Free light chain (FLC) kappa and lambda Assays for Atellica® NEPH 630*, BNTM II, and BN ProSpec® Systems are developed for more reliable managment of patients with monoclonal gammopathies. Improved diagnostic accuracy and sensitive detection of changes in follow-up testing are both aided by excellent and stable lot-to-lot repeatability.

N Latex FLC kappa and N Latex FLC lambda Assays. Image Credit: Siemens Healthineers
N Latex FLC kappa and N Latex FLC lambda Assays provide users with more confidence while screening and monitoring monoclonal gammopathies:
- Antigen-excess security is ensured by the pre-reaction protocol
- Monoclonal antibodies provide high specificity
- Reagents, supplemental reagents, standards, and controls are all available individually and can be mixed and matched as desired
- Excellent lot-to-lot reproducibility
Features and benefits
Consistent results help refine disease management strategies
Since 2009, the official recommended screening panel for multiple myeloma has been included in the determination of FLC. FLC determination also aids in the assessment of treatment progress.
For continuous high sensitivity in screening and precise identification of changes in follow-up, reliable findings are required. The clinical concordance of the N Latex FLC Assays to competitive assays has been demonstrated in studies.
How can improved lot-to-lot reproducibility and antigen-excess security improve the management of patients with monoclonal gammopathy?
N Latex FLC kappa and N Latex FLC lambda are two types of N Latex FLC. On Siemens Healthineers’ automated immunonephelometric devices, these assays provide an easy technique to determine free light chains.
Excellent lot-to-lot consistency
- Allows for patient screening and therapy monitoring, for example, myeloma patients
- Improves patient care by detecting possible relapses early and making appropriate therapeutic modifications, which can result in a better overall patient outcome
- Improves patient outcomes and patient follow-up while utilizing diverse reagent lots
Improved antigen-excess security
- Minimal dilution processes improve cost-effectiveness
- Detecting high-dose hook effects give users more confidence
Convenient packaging concept
- With tests and systems from Siemens Healthineers, users can be confident in their screening and monitoring of monoclonal gammopathies
- Flexible and reagent-independent packaging cuts expenses. All components can be bought individually, ensuring an adequate supply of standards and controls while reducing waste
Clinical use
A comparison of immunoassays to immunofixation electrophoresis (IFE), number of abnormal results:
Table 1. Source: Siemens Healthineers
| IFE, kappa Positive |
N Latex FLC kappa Assay |
FREELITE kappa |
| 60 |
60 (100%) |
59 (98.3%) |
| |
|
|
| IFE, lambda Positive |
N Latex FLC lambda Assay |
FREELITE lambda |
| 59 |
60 59 (98.3%) |
56 (94.9%) |
| |
|
|
| IFE, kappa and/or lambda Positive |
N Latex FLC Ratio |
FREELITE FLC Ratio |
| 115 |
105 (91.3%) |
103 (89.6%) |
Comparison of N Latex FLC assay and FREELITE FLC assay
(n = 1118, categorized as abnormal low, normal, abnormal high):
Table 2. Source: Siemens Healthineers
| |
Agreement rate |
Cohen’s coefficient |
| FLC kappa |
89.7% |
0.80 |
| FLC lambda |
82.4% |
0.67 |
| FLC ratio |
91.8% |
0.82 |
FLC ratio method comparison
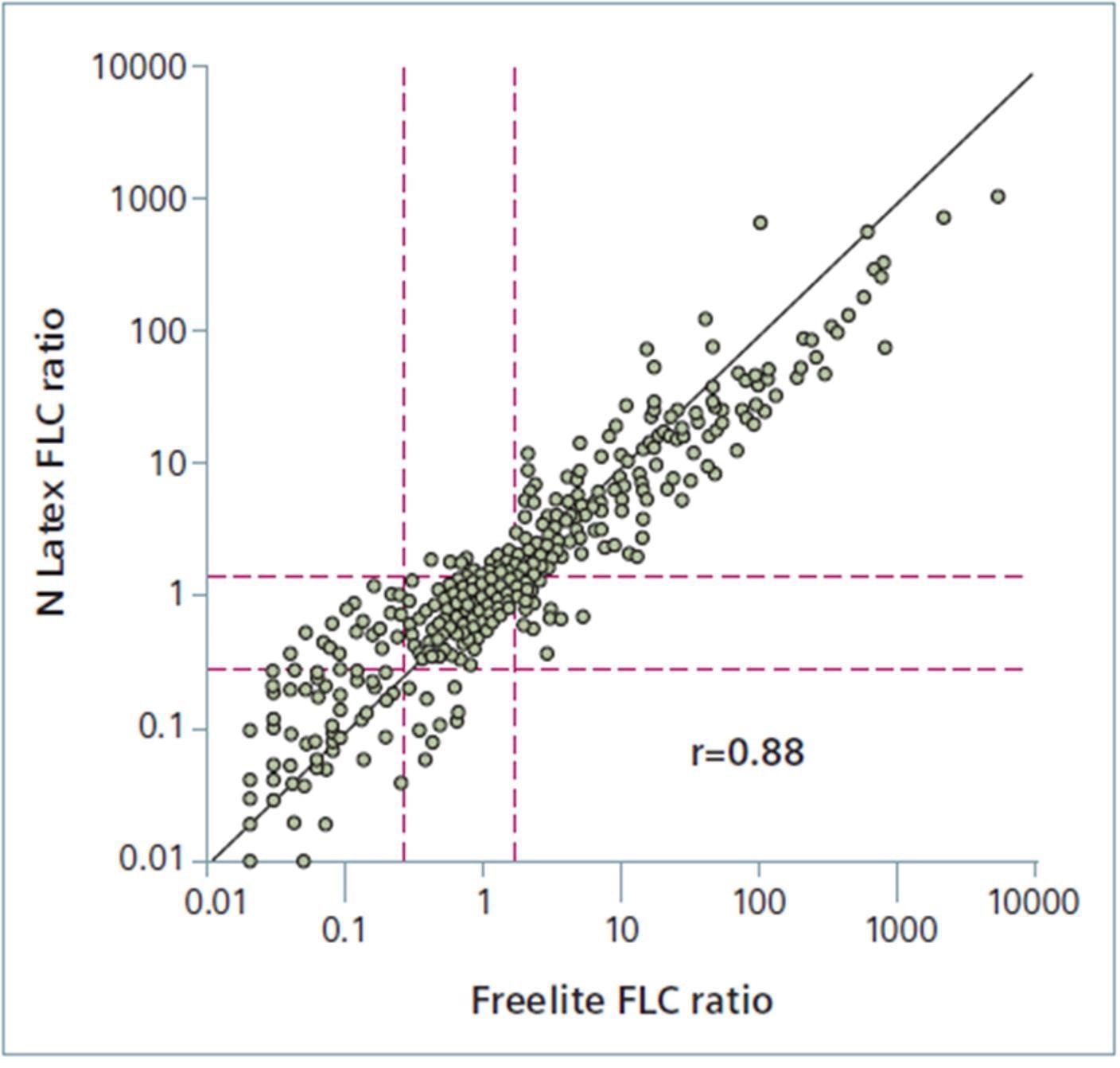
FLC ratio method comparison. Image Credit: Siemens Healthineers
Lot-to-lot comparison
Three distinct reagent lots were compared (n = 95), including two different (lot-independent) standard lots.
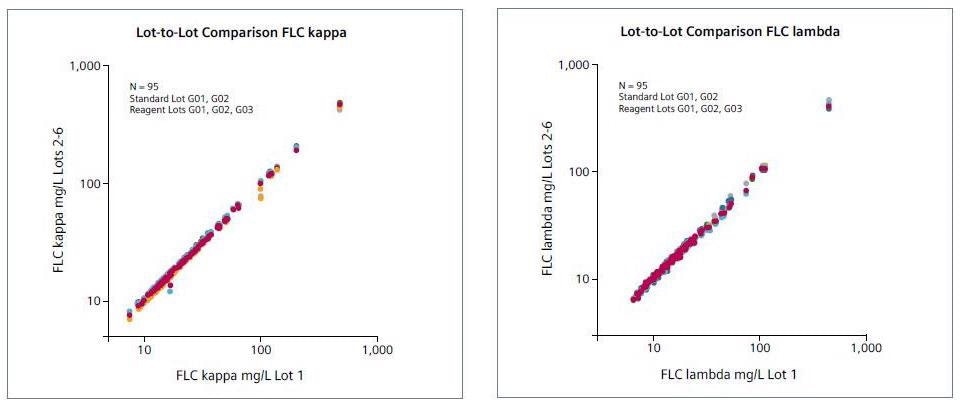
Image Credit: Siemens Healthineers
Technical specifications
Analytical assay performance
Table 3. Source: Siemens Healthineers
| . |
. |
| Assay principle |
Latex-enhanced immunonephelometry |
| Sample type |
Human serum, EDTA plasma, heparinized plasma†, CSF†, urine† |
| Reference range |
FLC kappa: 6.7 – 22.4 mg/L (US 8.24 - 28.9 mg/L)
FLC lambda: 8.3 – 27.0 mg/L (US 9.1 - 32.6 mg/L)
FLC ratio: 0.31 – 1.56 (US 0.53 - 1.51) |
| Initial measuring ranges |
3.5–110 mg/L for FLC kappa
1.9–60 mg/L for FLC lambda |
| Total measuring range |
0.17 – ≥ 2,000 mg/L for FLC kappa
0.47 – ≥ 6,000 mg/L for FLC lambda |
| Measuring time |
12 minutes |
| Onboard reagent stability |
2 weeks (Atellica NEPH 630* and BN ProSpec Systems)
5 days (6 days with evaporation stoppers) on BN II System |
| Calibration frequency |
6 weeks |
| Precision |
Repeatability: < 5%
Within-lab: < 6.5% |
| Antigen-excess security |
FLC kappa: up to 23,000 mg/L
FLC lambda: up to 57,000 mg/L |
*Not available for sale in the U.S.
†Applications are not available in the U.S.