With Thermo Scientific Handheld NIR Analyzers and Raman Spectrometers, pharmaceutical and biotechnology manufacturers can obtain quick, precise pharmaceutical analysis outcomes and raw material verification via plastic bags, blister packs, glass containers, and clear gel caps in around 30 seconds in the field to fight the spread of falsified medicines.
Developed for compliance with present good manufacturing practices (cGMP) and 21 CFR Part 11, the Thermo Scientific™ TruScan™ RM Handheld Raman Analyzer and the Thermo Scientific™ microPHAZIR™ RX NIR Analyzer can help users obtain high-quality pharmaceutical analysis and verification.
Identify and quantify pharmaceutical materials
Raw material testing and identity verification are vital steps in the quality control and pharmaceutical analysis process with enormous effects on customer safety as well as speed and cost of production.
The TruScan RM Handheld Raman Analyzer and microPHAZIR RX NIR Analyzer can be utilized instantly on the warehouse floor and at any pharmaceutical drug analysis point across the manufacturing process. Inspection intervals are increased, inventory management is enhanced, and supply chain risk is decreased globally.
The products take users beyond simple material ID, helping them to differentiate changing components in a mixture and quantify components in a material with the TruTools chemometrics functionality fixed in the TruScan RM Handheld Raman Analyzer. TruTools allows users to construct custom qualitative and quantitative techniques for complicated material analysis issues anywhere in the plant.
Users can make more informed decisions at the point of need, increasing productivity and pharmaceutical analysis efficiency. Utilize the TruScan RM Handheld Raman Analyzer with TruTools for at-line process monitoring and the pharmaceutical analysis process. Applications consist of endpoint determination of reaction monitoring, distillations, and component quantification of powder blended mixtures.

Image Credit: Thermo Fisher Scientific – Handheld Elemental & Radiation Detection
Identify counterfeit drugs
Falsified and substandard medicines are largely entering the supply chain, constituting a fatal and growing global health risk for patients and an expensive violation of intellectual property rights available for pharmaceutical manufacturers. Such drugs at best will fail to deliver the therapy that they promised and at worst could endanger the lives of patients.
The handheld Raman and NIR analyzers available for pharmaceutical identification and QC manufacturing have the potential to authenticate medication directly via sealed packaging, wherever pharmaceuticals have been distributed. These user-friendly tools allow a higher level of screening, decreasing the backlogs linked to laboratory testing.
Image Credit: Thermo Fisher Scientific – Handheld Elemental & Radiation Detection
End-to-end solutions for pharma and biotech
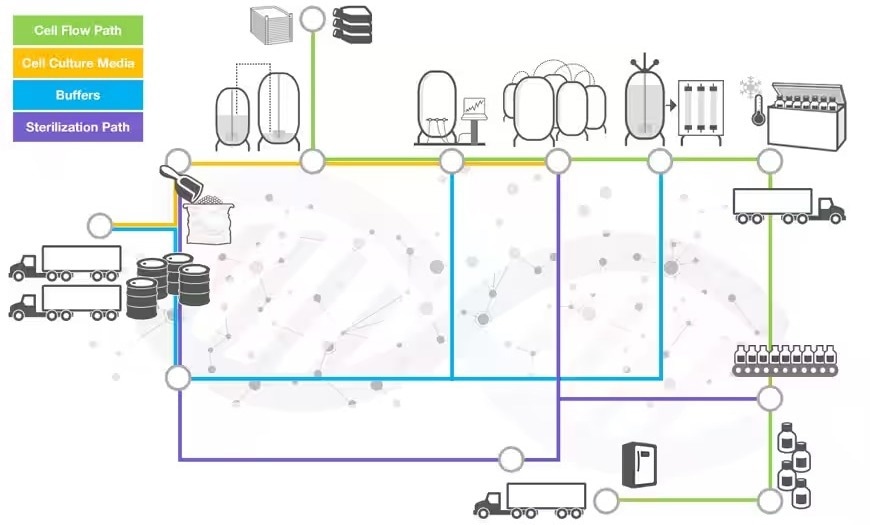
Image Credit: Thermo Fisher Scientific – Handheld Elemental & Radiation Detection