Taurine, also known as 2-aminoethanesulfonic acid, is a conditional amino acid that is found in natural dietary sources, biosynthesized in the body and produced by chemical synthesis for commercial purposes.
It was first isolated in 1827 by two German scientists, Friedrich Tiedemann and Leopold Gmelin, who discovered the presence of the substance in the bile of an ox. The name, taurine, is derived from the Latin term taurus, which means bull or ox.
Taurine is referred to as a conditional amino acid because it is derived from cysteine like other amino acids but lacks a carboxyl group that usually belongs to amino acids. Instead, it contains a sulfide group and can be called an amino sulfonic acid.
 Image Credit: Eugeniusz Dudzinski/Shutterstock.com
Image Credit: Eugeniusz Dudzinski/Shutterstock.com
Physiological functions
Taurine is found in high concentration in many parts of the body such as the eyes, central nervous system and skeletal muscles.
Taurine is thought to have a significant impact on the cardiovascular system and is a major reason taurine supplementation may be recommended. The cardiac muscles are strengthened in the presence of taurine, leading to improved overall function. This effect is also seen in skeletal muscles and is believed to improve exercise capacity and physical abilities.
Additionally, the inhibitory GABA receptors in the brain are activated by taurine. This leads to the assumption that taurine has an inhibitory effect on the brain pathways, stabilizing stimulation effects seen by other substances, such as caffeine.
Natural food sources
Taurine is naturally found in some food sources, such as eggs, milk, seafood and meat. The daily intake of taurine varies greatly between individuals, from 10 – 400 mg per day, with an average of 58 mg.
Individuals following a vegan diet tend to have the lowest intake levels, due to the animal-based sources of taurine.
Synthesis and production
Taurine is naturally synthesized in the pancreas of the human body, via a process called the cysteine sulfinic acid pathway. This involves the oxidation of the sulfhydryl group on the cysteine molecule to form cysteine sulfinic acid, which undergoes decarboxylation to form hypotaurine and eventually taurine.
As public consumer demand for taurine has increased, commercial production of the substance has become necessary, with the introduction of chemical synthesis.
This is usually done with a reaction between ethylene oxide and sodium bisulfite to form isethionic acid, which is used to obtain the synthetic form of taurine. Alternatively, a reaction between aziridine and sulfurous acid is a single reactive process that can be used to obtain taurine.
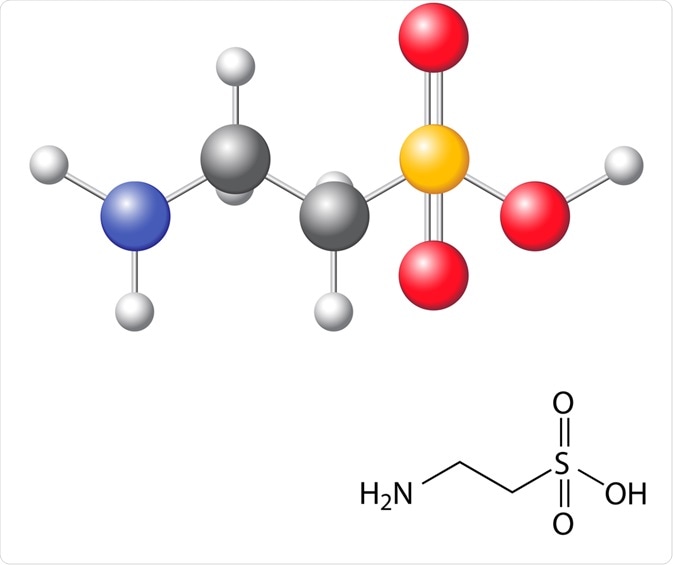 Image Credit: chromatos/Shutterstock.com
Image Credit: chromatos/Shutterstock.com
Use in energy drinks
Taurine is often included as an ingredient in energy drinks, which is most likely due to its physiological effect to improve muscular function and physical performance.
In comparison to the average dietary intake of 58 mg per day, many energy drinks contain high doses of taurine with 1000-2000 mg in each serving. Considering that some individuals may consume more than one serving each day, this has been a concern for some health advocates and stimulated research in the area.
However, doses of up to 3000 mg per day are generally considered to be safe with side effects rarely seen, although the long-term outcomes are not clear. It appears that the other constituents of energy drinks, such as glucose and caffeine, are more likely to cause significant side effects in high doses than taurine.
References
Further Reading
Last Updated: Mar 12, 2021