Chronic hepatitis C infection is one of the most frequent chronic infectious diseases worldwide. The WHO estimates that 150 million people worldwide are chronically infected with the hepatitis C virus (HCV).
40-50 million people are thought to be mono-infected with HIV. The overlap, i.e. those that are infected with both HCV and HIV, is probably 8-10 million people worldwide.
If you look into certain subgroups like IV drug users, even up to 90% of HIV patients are co-infected with HCV.
So HCV/HIV co-infection is a huge problem worldwide.
Why is there a high unmet medical need for these patients?
The medical need is high because the HIV infection background accelerates liver disease progression in patients who are co-infected with HCV.
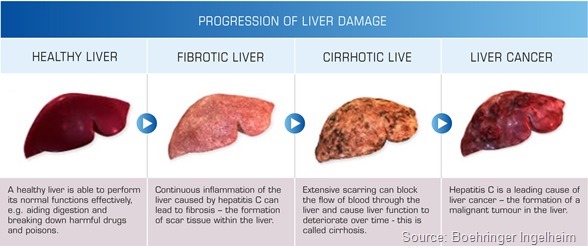
This is probably due to constant inflammation and inflammatory processes in the liver and possibly due to the immune defects in HIV patients. So those patients develop liver cirrhosis more frequently and quicker than HCV mono-infected patients.
Since HIV treatment has become so successful, liver disease, and HCV being the lead cause of liver disease, has now become one of the leading mortality reasons in HIV infections. So there is indeed a very high need.
On the other hand it is very difficult to treat these patients because they have a lower treatment response, at least with interferon-based treatments in the past. This was probably due to the immune defects caused by the HIV virus.
Also many patients take HIV drugs which leads to drug interactions with any co-medications including the new hepatitis C treatments.
HIV patients have more side effects – not only because of the co-medication – but for whatever reason, probably due to chronic inflammation due to the HIV. They experience more severe side effects to any kind of treatment like antibiotics and so forth. This makes any drug treatment more burdensome in HIV patients.
It is clear that these patients are more difficult to treat and on the other hand they have a higher need to be treated. We hope that the new compounds in development, such as faldaprevir, will reduce the burden of treatment that is currently caused by the first generation protease inhibitors that are on the market.
What factors influence the likelihood of treatment success in HCV and HIV co-infected patients? How do these factors differ from HCV or HIV mono-infected patients?
In general all factors that are associated with treatment success or cure in mono-infected patients also apply to HIV infections.
The list is quite long, it includes:-
- The virus type – genotype 1 or other HCV genotypes
- The subtype of the virus – subtype 1A being more difficult to treat than subtype 1B
- Baseline factors such as the viral load, gender, the duration of treatment, the stage of liver disease
- The host’s genetics – there are polymorphisms in the whole genome that are positively or negatively correlated with treatment response.
In addition to these factors HIV patients have further factors impacting the treatment response. These include the stage of HIV disease with patients having an inferior immune status – having a lower response to current treatments.
Also we must remember there are compliance issues. Many HIV patients have to take 1-3 HIV drugs per day several times a day depending on their regimen. This leads to compliance issues as they don’t like to take additional treatments on top of this.
Finally drug-drug interaction is also problematic in HIV patients. Some drugs cannot be taken together with HCV treatments. Any treatment decision needs to be tailored to the patients. You need to pick the right treatment for your patient profile. This is how we see the future of HCV treatments.
Please can you outline the aims of the STARTVersoTM 4 study?
Our interferon based phase III program is called the STARTVersoTM program. It is where we approach all relevant genotype 1 HCV populations. The HCV/HIV co-infected patients are a very important population for us, as we have a very strong HIV heritage in Boehringer Ingelheim.
This is part of a very comprehensive study program and the aim of the study is to characterise the safety and efficacy of our compound faldaprevir on a background of HCV/HIV co-infected patients.
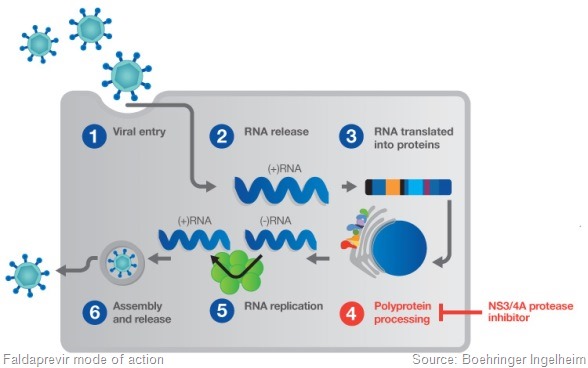
We need to understand which the best dose regimen is. We’ve been testing two doses of faldaprevir – the 120mg and 240mg doses. We’re testing two durations.
We are also testing whether any response-guided treatment options are a possibility not only in HCV mono-infected patients but also in HIV co-infected patients. That means that patients who achieve an early response criteria, which we call ETS in our trial, can stop any treatment after 24 weeks – so basically they can cut short the overall treatment cycle to only half of the previous treatment duration.
One of the aims of this trial, which is the largest HCV/HIV co-infected trial that has been reported so far, is that we want to be able to recommend a regimen that provides the best risk/benefit ratio to a given HCV/HIV patient.
Why is the potential for a shorter treatment duration for HCV/HIV co-infected patients important?
These patients are tired of taking drugs because they also take HIV drugs. They are more susceptible to severe side effects of treatment. We want to keep the burden of treatment that is imposed on these patients by PegIFN/RBV plus the protease inhibitor as short as possible as for any other patient.
In addition, the compliance issues I previously mentioned are even stronger in HCV/HIV co-infected than in mono-infected patients.
What kinds of patients are being treated in the STARTVersoTM 4 study?
We are evaluating both HIV and HCV patients. The HIV patients are either on no therapy or on a stable HIV treatment.
With regards to the HCV condition we are evaluating genotype 1 patients. We are evaluating those who have never received HCV treatment before as well as patients who have had treatment in the past which they have responded to but are now undergoing a so-called relapse.
This is a really difficult to treat population. If you look at the baseline demographic in the study, 17% of patients had liver cirrhosis already and nearly 80% had the difficult to treat genotype 1A.
We are covering a rather broad HCV/HIV co-infected population here including very difficult to treat populations.
How is the study going so far?
The study has enrolled rather quickly and we presented the first interim data at the Conference on Retroviruses and Opportunistic Infections (CROI) earlier this month.
The important messages from our trial are clearly with regards to the efficacy that the early on treatment activity seems to be very comparable to the efficacy we see in HCV mono-infected patients with 80% of patients roughly achieving an early treatment success. So if response-guided treatment turns out to be working, then 80% of patients would require only half a year of treatment. We obviously need to wait for the full data – but this is very promising so far.
It is very important to note that none of the patients have lost their HIV suppression, so there was no negative effect on the HIV infection and the adverse event profile looked comparable to what we see in our regimen for mono-infected patients even though we need to be cautious here because we do not have a control arm in this trial.
But overall it is looking very promising and we are excited about the results.
How do the safety results of the study compare to those observed in HCV mono-infected treatment-naïve patients in prior faldaprevir clinical studies?
There is nothing new in the AE profile due to faldaprevir, however, we cannot comment on the frequency of specific events because there is no control group in this trial. However, the overall picture looks quite comparable to any PegIFN/RBV treatment.
When are the final trial outcomes likely to be presented?
The final outcomes will be by the end of this year so these will be presented at one of the major conferences early next year.
Where can readers find more information?
World Hepatitis Alliance: https://www.worldhepatitisalliance.org/
European Liver Patients Association: http://www.elpa-info.org/
About Professor Wulf Boecher
 Wulf trained and worked more than 15 years at Mainz University Hospital in Germany as a Gastroenterologist/ Hepatologist and Infectious Diseases Specialist, where he lectures as an Associate Professor for Internal Medicine.
Wulf trained and worked more than 15 years at Mainz University Hospital in Germany as a Gastroenterologist/ Hepatologist and Infectious Diseases Specialist, where he lectures as an Associate Professor for Internal Medicine.
His main scientific focus has been immune pathogenesis and new treatments of HBV, HCV and HIV infection.
Wulf joined Boehringer Ingelheim for the clinical development of new HCV treatments in 2007 and currently holds the position of Associate Therapeutic Area Head Virology.