As surgeons who take care of cancer patients, we have observed a troubling association between patients who suffer from infections after surgery and the rapid spread of their cancer. We have reported this in lung cancer patients, and others have made these observations in a variety of different cancers. However, the mechanisms responsible for this association are still poorly understood.
What we do know is that in response to infection, the number of white blood cells, particularly neutrophils detectable in the blood increases in order to fight infection. Studies that have shown high levels of neutrophils in cancer patients are associated with increased rates of metastasis.
While interesting, this is just an observation and by no means can we conclude that high levels of neutrophils cause metastasis. We have made similar observations in the lab and reported last year a study highlighting these findings and which lead directly to this present one.
By performing microscopy of the liver in living mice, we are able to visualize interactions between tumor and immune cells, most notably neutrophils. What we noticed is that in mice with infections, tumor cells often get stuck in distant organs near neutrophils.
To determine if neutrophils play an active role in cancer spread to distant organs, we depleted them in mice with infections. What we found is that in the absence of neutrophils, mice are unable to form liver metastases. If we give mice back their neutrophils, their tumors regain the ability to form metastases. This implies a causal role for neutrophils in metastasis.

Dr. Lorenzo Ferri (left) and Dr. Jonathan Cools-Lartigue (right) – credit: Pierre Dubois, McGill University Health Centre (MUHC).
Please can you outline the mechanism by which this process occurs?
There appear to be many mechanisms by which this process occurs. In the presence of infection, neutrophils increase the number of molecules on their surface responsible for sticking to blood vessels throughout the body. White blood cells need to do this in order to get to sites of infection, where they kill invading pathogens.
However, cancer cells are also able to bind to these molecules. This means that they tend to aggregate in places where there are many white blood cells. By taking advantage of the ability of white blood cells to traffic to sites of infection, tumor cells may be able to gain access to distant organs not normally accessible to them.
The results of our study suggest that cancer cells are also able to bind to another, recently described, process through which neutrophils capture and destroy bacteria, termed Neutrophil Extracellular Traps, or NETs. These are webs of DNA that are released by neutrophils in response to infections. In the case of severe, disseminated infections, neutrophils throughout the body, not just at the site of infection can be stimulated to produce NETs. These NETs are usually used by the body to capture and destroy bacteria, and thus represent a protective function for the body.
However, we have found that cancer cells can be captured by these NETs, and rather than killing them, the cancer cells are made more active, and more likely to form secondary cancers, or metastasis.
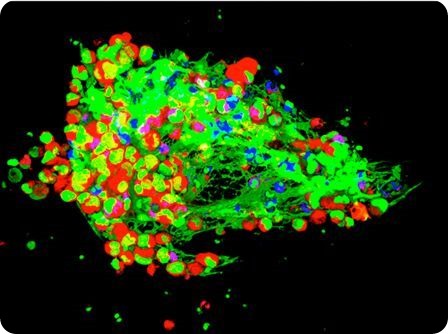
Cancer cells (in red) being trapped by NETs (in green) developed by white blood cells (in blue) – credit: Drs. Jonathan Cools-Lartigue and Lorenzo Ferri.
Why do you think this cancer spreading mechanism has never previously been identified?
Neutrophil extracellular traps have recently been described and were only discovered in 2004. Since then, a great deal of research has focused on their physiologic role in trapping and killing bacterial invaders. In fact, our collaborators in Calgary are leaders in this field of research.
Our particular interest in the link between severe infection and cancer spread led us to look at NETs from a different perspective. After seeing their role in infection, to us, the next logical question was; if NETs trap bacteria in the circulation can they trap cancer cells in the same way?
Your research was largely carried out on mouse models of cancer. Do you think your findings will translate in humans?
This is an excellent question and unfortunately we wont have a definite answer until we and others validate these results in humans.
What we do have now is circumstantial evidence suggesting these processes occur in humans. First, people who suffer from severe infections, at least post-operatively seem to have a higher risk of cancer spread.
Second, NETs have been demonstrated in humans in a variety of infectious states. Finally, early clinical reports are starting to surface suggesting that the presence of NETs in humans with cancer is associated with worse outcomes.

Dr. Lorenzo Ferri is a researcher at the RI-MUHC and an Associate Member of the Rosalind and Morris Goodman Cancer Research Centre in Montreal – credit: Pierre Dubois, McGill University Health Centre (MUHC).
How do the results of your study change the way in which we think about cancer progression?
The results of our study highlight the potential importance of the early post-operative period in the treatment of cancer. While surgery is an essential part of cancer therapy it does have its disadvantages, particularly infectious complications. Today, patients who suffer a post-operative infection have their chemotherapy delayed until they recover. In addition, no therapy exists to mitigate the consequences of infection on tumor spread and progression.
Our study highlights a novel mechanism by which cancer cells can take advantage of our immune system for their benefit after becoming trapped within NETs.
More importantly though, we show that NETs are a potential therapeutic target in cancer care. By disrupting them in a mouse model of lung cancer, we were able to limit metastasis formation after an infectious insult.
What impact will this research have on the diagnosis and treatment of cancer patients?
Our research has important implications in cancer treatment by highlighting the post-operative period as an opportune time to offer systemic therapy in the hopes of preventing cancer spread.
Unfortunately infections arise in up to 30% of patients undergoing cancer surgery, this thus represents a large cohort of patients at risk for this mechanism of cancer metastasis.
Ideally we would be able to identify patients at high risk for post-operative infections and we can interfere in the formation of NETs either before they develop, or early after they start to develop.

Dr. Lorenzo Ferri (left) and Dr. Johatna Cools-Lartigue (right) are looking at a human stomach cancer specimen – credit: Pierre Dubois, McGill University Health Centre (MUHC).
It has been stated that existing medications for non-cancer diseases may be able to be used to prevent the spread of cancer due to the white blood cell mechanism. Please can you tell us more about these medications and how likely do you think it is that they will be able to be used for the treatment of cancer metastasis?
Neutrophil extracellular traps are DNA webs decorated in antimicrobial proteins. The medications we used in this study were DNAse, a compound that breaks down DNA, and a small molecule inhibitor of a protein called neutrophil elastase. DNAse breaks down NETs after they are formed while the neutrophil elastase inhibitor prevents neutrophils from forming NETs in the first place.
DNAse is currently used in the treatment of cystic fibrosis and a lot of clinical experience exists with this medication. The neutrophil elastase inhibitor was originally designed for the treatment of patients with chronic lung diseases. While it is not used in humans to the same extent as DNAse, early clinical studies have been carried out.
We selected these medications in part because of the potential for their use in humans. We felt this would make any results we found more relevant to the clinical community. While the results of our study are exciting, they will need to be validated in humans before any of these medications can be used in the clinic.
What further research needs to be done to validate this?
I think we need to demonstrate a few things before any concrete claims regarding the role of NETs in human cancer progression can be made.
First we need to find a reliable test that demonstrates the presence of NETs in people. Second we would need to demonstrate a clear association between NET formation in cancer patients and higher than expected rates of metastasis.
If these two objectives are clearly met, clinical trials with medications targeting NETs could begin. Because extensive clinical experience with DNAse exists for example, such clinical trials could be started more rapidly than if we were to employ entirely new untested compounds.
How do you think the future of our knowledge of cancer progression will develop?
Cancer research has historically focused on factors that are intrinsic to cancer cells themselves. For example, what genes are involved, what proteins are expressed on the cancer cells themselves that make them more or less lethal. This perspective is reflected in modern chemotherapy, which exploits the cellular machinery of cancer cells to kill them.
While this research has brought tremendous gains in cancer care, more recent research has focused on the interactions between cancer cells and the normal surrounding cells. These interactions, including those with the immune system are increasingly being shown to be important and can affect whether cancer spreads or not.
As our understanding of these interactions matures, we may begin to see therapies targeting them emerge in the clinical care of cancer patients.
Where can readers find more information?
Our full study is available online and is entitled “Neutrophil Extracellular Traps Sequester Circulating Tumor Cells and Promote the Development of Metastasis” in the Journal of Clinical Investigation.
About Drs. Lorenzo Ferri and Jonathan Cools-Lartigue
 Dr Ferri is the director of thoracic surgery and holder of the David S. Mulder Chair in Surgery at McGill University in Montreal.
Dr Ferri is the director of thoracic surgery and holder of the David S. Mulder Chair in Surgery at McGill University in Montreal.
Dr Cools-Lartigue is a general surgery resident and PhD candidate in Dr Ferri's laboratory within the McGill University Health Centre Research Institute.