Mar 19 2015
BIOASIS TECHNOLOGIES INC. (OTCQX:BIOAF; TSX.V:BTI), a pioneering biopharmaceutical company focused on overcoming the limitations of therapeutic drug delivery across the blood-‐brain barrier (BBB), announces the results from an animal ischemic stroke model performed at the National Research Council Canada with the biOasis Transcend carrier peptide, MTfp and siRNA (MTfp-‐siRNA). Two key therapeutic effects were shown with MTfp-‐siRNA; A high degree of reduction of the area of blood vessel infarct and improvement of overall brain activity as determined by neurological scoring.
On July 25th, 2014, biOasis announced that its newly discovered carrier peptide (MTfp), part of the biOasis Transcend family of carrier technologies, effectively delivered siRNA across the BBB and into brain cells. The company also announced that it achieved the goal of silencing the expression of a selected target gene in the brain by approximately 50%. This study prompted the company to move to a model of disease where therapeutic effects could be measured.
Ischemic stroke is the most common type of stroke in humans and an animal model that mimics stroke was chosen to establish the effectiveness of MTfp-‐siRNA treatment administered immediately prior to induction of ischemic stroke.
Key Findings of Ischemic Stroke Model Treated with MTfp-‐siRNA:
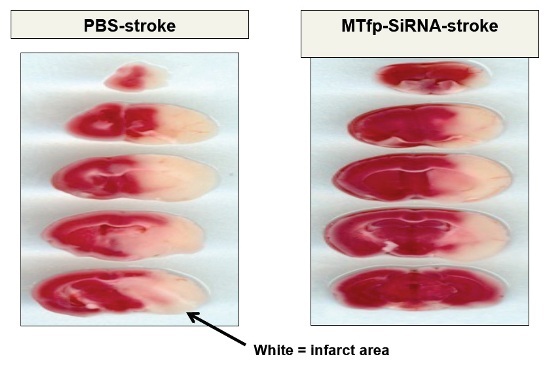
(1) The area of infarct (damage) was highly reduced in the animals treated with MTfp-‐siRNA. Brain images revealed significantly less stroke damage in brain sections from animals treated with the MTfp-‐siRNA when compared to the same brain regions in the control animals. The brain section images may be viewed below. The images show that there is more stroke damage (white areas) in the five sections from control animals (Left Panel) when compared to the same brain regions in the animals with stroke treated with the MTfp-‐siRNA (Right Panel).
(2) Thirty-‐minutes after induction of stroke, the neurological scores of the animals treated with the MTfp-‐siRNA were significantly better than those of control animals. At 24 hours, controls animals demonstrated a slight improvement in neurological scores while MTfp-‐siRNA treated animals showed a vast improvement and exhibited nearly normal neurological scores.
“The results clearly demonstrate that in an established animal model of stroke, therapeutic levels of siRNA that down-‐regulate the expression of a key pathogenic gene were successfully delivered by MTfp to the brain, resulting in a significant reduction of infarct damage and improvement of neurological scores associated with healthy brain function,” said Dr. Wilfred Jefferies, biOasis Founding Scientist.
CEO Rob Hutchison added:
biOasis is the first company to show delivery of siRNAs to the brain of living animals. Our findings open the way to development of new strategies for therapeutic interventions for a variety of brain disorders.
Brain Section Images