Otsuka Pharmaceutical Co., Ltd. announced that the European Commission has granted marketing authorisation for JINARC® (tolvaptan) for the treatment of ADPKD in adults who have chronic kidney disease (CKD) stage one to three at initiation of treatment with evidence of rapidly progressing disease. In receiving this marketing authorisation, tolvaptan becomes the first pharmaceutical therapy to be licensed in Europe for the treatment of the underlying pathophysiology of ADPKD.
Professor Ron T. Gansevoort, University Medical Centre Groningen, the Netherlands, an expert in the field of polycystic kidney disease said:
Until now, healthcare professionals have focused on treating the signs and symptoms of ADPKD, with no specific treatment available to treat the disease,” Tolvaptan represents a significant medical breakthrough in the management of ADPKD. For the first time, healthcare professionals can modify the progression of the disease and preserve kidney function, with the potential to improve patients’ quality of life and long-term outcomes
The marketing authorisation for tolvaptan is based on the findings of the pivotal Phase III randomised, double-blind and placebo-controlled TEMPO 3:4 trial – the largest clinical study conducted in ADPKD to date. In the three-year study, the rate of TKV increase over 3 years was significantly less for tolvaptan-treated subjects than for subjects receiving placebo: 2.80% per year vs 5.51% per year, respectively (ratio of geometric mean 0.974; 95% CI 0.969 to 0.980; p <0.0001); these data demonstrate an approximate 50% significant reduction in the annual increase in TKV versus placebo.
Furthermore, tolvaptan showed a statistically significant reduction in the risk of multiple events of worsening kidney function, kidney pain, hypertension or albuminuria (hazard ratio=0.87, 95% CI: 0.78-0.97, p=0.0095). The result of the key secondary composite endpoint is primarily attributed to effects on worsening kidney function (61.4% less likely with tolvaptan than with placebo) and medically significant kidney pain (35.8% less likely in tolvaptan-treated patients).
Other than side effects associated with the mechanism of action of tolvaptan (eg thirst, polyuria, polliakuria), most side effects observed in ADPKD patients administered tolvaptan were comparable with those administered placebo. However, a risk of liver injury was identified in patients with ADPKD taking tolvaptan. Elevation of alanine transaminase (ALT) was observed in 4.4% of patients on tolvaptan and 1.0% of patients on placebo. Two (2/957, 0.2%) tolvaptan treated-patients, as well as a third patient from an extension open label trial, exhibited clinically significant increases in ALT with concomitant elevations in total bilirubin (BT).
While these concomitant elevations were reversible with prompt discontinuation of tolvaptan, they represent a potential for significant liver injury and patients taking tolvaptan will have to undergo monthly blood tests for the first 18 months of treatment with tolvaptan and three-monthly thereafter to mitigate this risk. Tolvaptan treatment must be initiated and monitored under the supervision of physicians with expertise in managing ADPKD and a full understanding of the risks of tolvaptan therapy including hepatic toxicity and monitoring requirements.
Tess Harris, President of PKD International said:
The progressive and hereditary nature of ADPKD is a physical and emotional burden on those living with the condition, as well as their families and loved ones, This approval is welcomed by the ADPKD community as it represents a step forward for the thousands of patients and carers throughout Europe who are affected by the disease
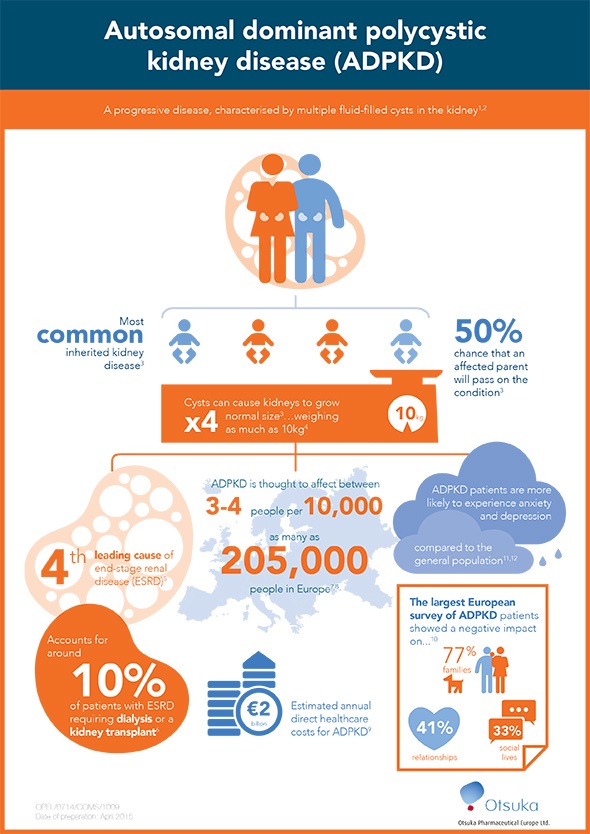
ADPKD is the most common inherited kidney disease primarily characterised by the proliferation and growth of multiple fluid-filled cysts in the kidney. Cyst growth and expansion in both kidneys leads to slow deterioration of kidney function, and approximately half of patients reach end-stage renal disease (ESRD) and require renal replacement therapy (RRT) in the form of dialysis or a kidney transplant by the age of 54. ADPKD is the fourth leading cause of ESRD in adults and accounts for around 10% of patients requiring RRT.
Tatsuo Higuchi, President and Representative Director of Otsuka Pharmaceutical Co., Ltd said:
It is a great honour to deliver the first treatment for ADPKD in Europe, this approval is testament to the invaluable endeavours of the researchers and patients involved in the discovery and development of tolvaptan
Tolvaptan was first approved for patients with ADPKD in Japan in March 2014 and was approved for ADPKD in Canada in February 2015. Following this European marketing authorisation, Otsuka will continue to work with local authorities in countries throughout Europe to help ensure that eligible ADPKD patients are able to access tolvaptan.
Source:
Otsuka Pharmaceutical Co., Ltd.