Aug 8 2016
Boosting levels of a protein that controls the shape and activity of a crucial group of white blood cells improves survival and recovery chances during sepsis.
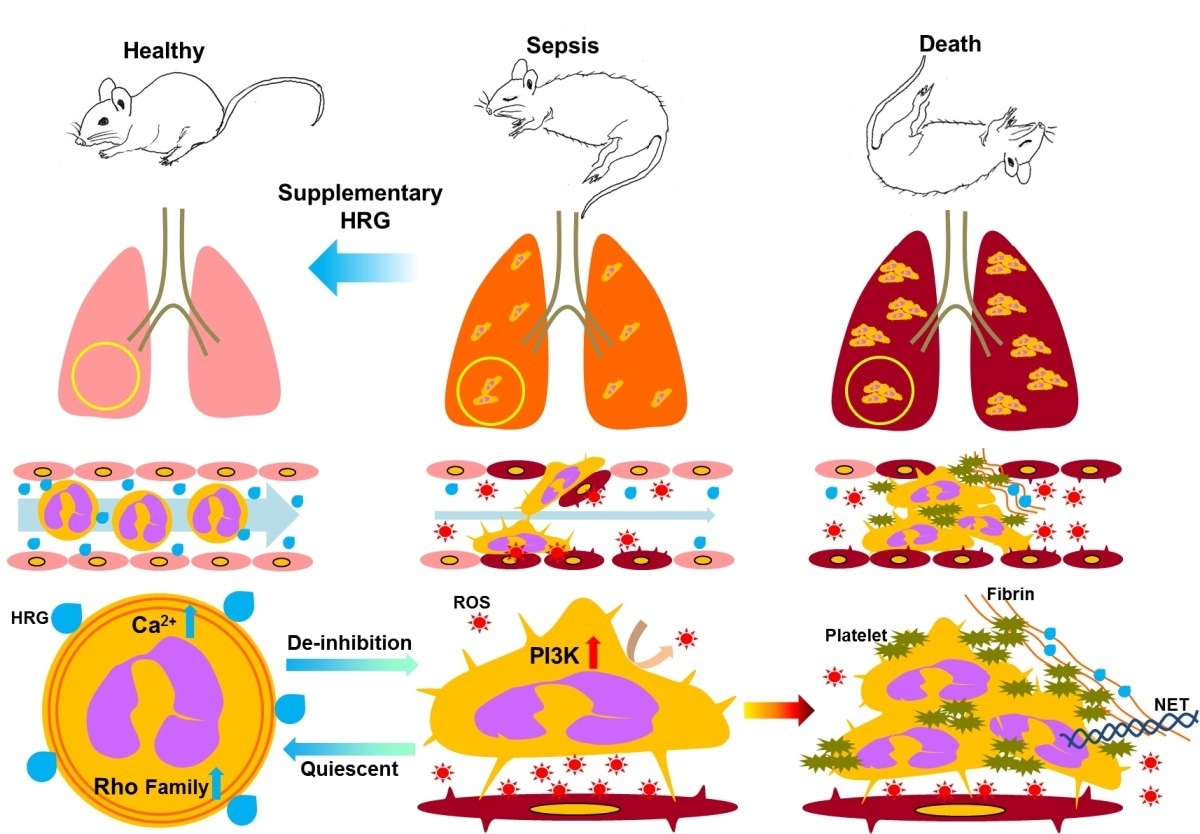
Novel Understanding of Sepsis Physiology and a Proposal of New Treatment Strategy
Decrease in plasma HRG in septic condition triggers the deformation of circulating neutrophils and the tight attachment of these cells to vascular endothelial cells, associated with ROS production and endothelial cell injury . This in turn facilitates the NETosis, platelet aggregation and coagulation, leading to immunothrombosis and multiple organ failure.
Blood poisoning following an infection or injury is known as sepsis, and is a major cause of death across the world. Sepsis occurs when the body’s immune system goes into overdrive, resulting in damage to its own tissues and organs through insufficient blood supply. However, the exact molecular mechanisms underpinning sepsis and its progression are unclear.
Now, Professor Masahiro Nishibori and co-workers at Okayama University, Shujitsu University and Kinki University, Japan, have shown that a naturally-occurring protein called histidine-rich glycoprotein (HRG) plays a significant role in the prevention of sepsis. They found that HRG controls the shape and activity of white blood cells called neutrophils, enabling them to flow freely and respond correctly in the fight against sepsis.
Nishibori’s team aimed to verify the role of HRG – a protein produced and secreted by the liver - because HRG levels decrease rapidly in patients when sepsis takes hold. HRG is known to be involved in the regulation of immune responses, as well as prompting antibacterial and antifungal activity. The team induced sepsis in one group of mice, keeping a healthy group as controls. They purified HRG from human blood plasma, and treated some of the septic mice with a dose of the protein.
The researchers found that the HRG mice quickly regained locomotor activity, and began to recover from sepsis. Further investigations showed that the mice exhibited far less inflammation in the lungs than their non-treated counterparts. Neutrophils in the HRG mice were smooth and spherical in shape, allowing them to flow freely through microcapillaries and veins. The septic mice, however, had deformed neutrophils with irregular shapes. This in turn triggered unwanted activity because the deformed neutrophils became attached to other cells, creating cell clusters that limited blood flow.
“The decrease in plasma HRG constitutes the fundamental pathway for septic pathogenesis,” state the authors in their paper published in eBioMedicine (2016). “Supplementary therapy with HRG may provide a novel strategy for the treatment of septic patients.”