Oct 19 2016
Helicobacter pylori is a spiral bacterium that can colonize the human stomach – sometimes with fatal consequences. A research group led by Prof. Markus Gerhard of the Technical University of Munich (TUM) and Assistant Professor Dr. Bernhard B. Singer of the Institute for Anatomy at the Faculty of Medicine of the University of Duisburg-Essen at Essen University Medical Centre has discovered a completely new approach to preventing or treating infections with this bacterium as well as secondary complications. This research was done in collaboration with the group of Prof. Han Remaut (VIB – VUBrussels, Belgium). Scientific journal "Nature Microbiology" reports on this in its current edition.
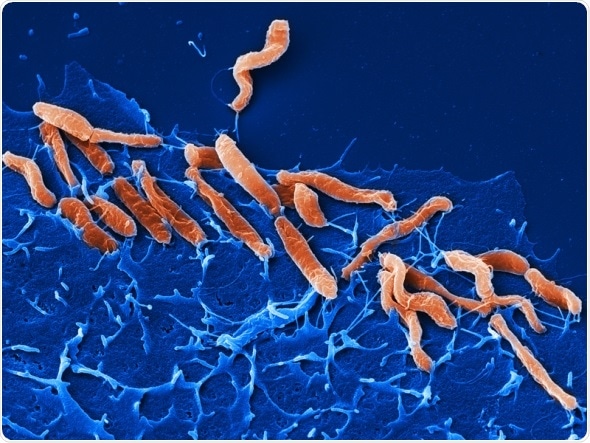
Helicobacter pylori infection increases the risk of developing stomach cancer.
(Image: M. Rohde/HZI)
Helicobacter pylori infection usually occurs during childhood. The bacterium is widely spread: every third person in Germany and every other person worldwide is a carrier. Secondary complications include gastritis, stomach and duodenal ulcers. In addition, there is an increased risk of developing stomach cancer. The typical treatment for Helicobacter pylori infections is currently antibiotics. The disadvantage of this treatment, however, is that it not only destroys the bacterium itself but also the ‘good germs’ of the gut flora. In addition, the bacterium is developing increasing resistance.
In order to ensure permanent survival in the human stomach, Helicobacter pylori must attach to the epithelial cells in the gastric mucosa. Research groups in Munich, Essen, and Brussels have now detected a highly specific and exceptionally strong variant of this adhesion, in which the bacterial surface molecule HopQ binds itself to so-called "Carcinoembryonic Antigen-Related Cell Adhesion Molecules", or CEACAMs for short, inside the stomach.
Independent of sugar
"In contrast to previously known binding partners of the bacterium, this bond is independent of sugar structures. This seems to ensure that it is especially stable in the acid environment of the stomach," explains Bernhard B. Singer. CEACAMs do not occur in healthy stomach tissue, but primarily when there is an inflammation of the gastric mucosa (gastritis) caused by Helicobacter pylori infection.
"One could say that these germs create additional and particularly strong binding opportunities by stimulating the formation of CEACAMs," adds Singer. Once bound to CEACAM, Helicobacter pylori can transfer additional proteins, so-called virulence factors, to the stomach cells. This secretion system contributes significantly to the development of stomach ulcers and bowel cancer. "Against this backdrop, we assume that HopQ could be used diagnostically and therapeutically," says Markus Gerhard, Professor at the Institute for Medical Microbiology, Immunology and Hygiene at TUM.
New approaches to treatment
The Scientists are currently researching various approaches in order to replace current types of treatment for Helicobacter pylori infection, due to the aforementioned side effects. The adhesion of the bacterium to stomach cells could be prevented with a soluble version of HopQ or parts of the protein, and the damaging effects of the germ could potentially be suppressed, as the data in the publication indicate. As a further therapeutic option, the researchers are pursuing the approach of using specifically developed antibodies against CEACAMs in order to fight diseases associated with the bacterium. An additional treatment option being considered is immunization against the HopQ protein and thus vaccination against infection with the bacterium.
The German Research Foundation (DFG) considers the project a promising approach and will be sponsoring further research over the next three years. Results by a group led by Prof. Wolfgang Haas of Ludwig-Maximilians-University’s Max von Pettenkofer-Institute confirm the data collected by Markus Gerhard and his colleagues. Both articles appear in the current issue of "Nature Microbiology".