An interview with Máté Marosi, Ph.D., and Zsolt Iván, PharmD., discussing the use of acousto-optic microscopes to study neural networks in the brain, from cell bodies to dendrites, to tiny spines. This interview was conducted at the Society for Neuroscience annual conference in 2019.
Why do scientists want to visualize neural networks plane-by-plane?
Máté: To start with, let’s look at why scientists want to visualize neurons. Electrophysiological techniques allow us to study neural signaling both at the single cell (limited cell numbers at the same time) and network level. However, at the network level, we cannot distinguish the spatial distribution of the signals (at the cellular level).
To get access to the smallest elements of the brain, desires to do so in the largest scanning volume possible, with the highest temporal and spatial resolution they can achieve. Multiphoton microscopy seems to be an ideal tool.
Scientists want to capture neuronal activity, not just on a single cell level, but across an entire neural network simultaneously.
However, conventional 2-photon imaging systems operate mainly in a 2D plane, but neuronal networks are dispersed in three-dimensional space. These microscopes allow you to see up to hundreds of neurons with limited temporal frequency, but this is only a tiny snapshot of the neuronal network.
In addition, neuronal networks are organized in 3D. So being able to understand the proper role of the neurons in the entire network would be much more powerful. Our new innovation is allowing neuroscientists to follow the activity of thousands of neurons in a three-dimensional brain region.
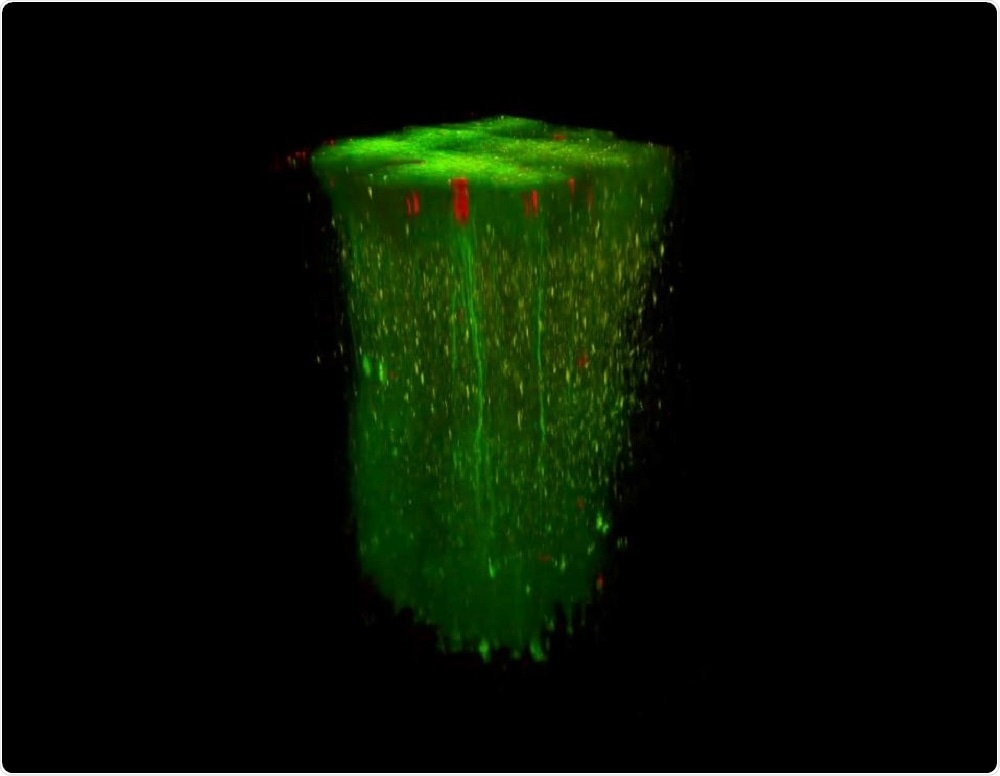 In vivo 3D recording in large volumes. Image Credit: Femtonics.
In vivo 3D recording in large volumes. Image Credit: Femtonics.
How are Femtonics changing the game when it comes to multiphoton imaging?
Máté: The understanding of brain computations requires methods that read out neural activity on different spatial and temporal scales. Moreover, fast recording is required not only from a single plane or point, but also at the level of large neuronal populations situated in large 3D volumes.
Femtonics developed an acousto-optic (AO) technology-based 2-photon microscope called the Femto3D Atlas (the peak of the evolution of Femtonics microscopes) that provides real-time 3D imaging and photostimulation. It allows scientists to observe simultaneous neuronal activity on a cellular or even subcellular (dendritic or dendritic spine) levels across multiple planes. We can also arbitrarily rotate our imaging planes and find the best angle for the actual recording.
The technology also allows us to only scan the structures from the regions of interest to perform measurements at a higher speed. You can visualize cell bodies or dendrites at a high temporal and spatial resolution for fast readout of neuronal and network activity in 3D. That’s what makes this system unique.
Zsolt: Our state-of-the-art technology is a pioneer on the field of two-photon microscopy. It comes with a powerful temporal and spatial resolution which is also combined with unique methods in 3D imaging and photostimulation. Our innovation is not only groundbreaking but completely, changing the game when it comes to optical laser scanning multiphoton microscopy.
How does the Femto3D Atlas work, and where did the idea come from to use an acousto-optic deflector?
Máté: The description of physics behind the acousto-optic technique dates back to early twentieth century, but it was not used for biological purposes until the early 2000s
Zsolt: Around 15 years ago, founders of Femtonics - who are also scientists - recognized the potential of the acousto-optic technique for neuroscience.
Máté: So, the microscope works by shooting a laser through special crystals (acousto-optic deflectors). The phrase “acousto-optic” refers to the field of optics that studies the interaction between sound and light waves.
Acousto-optical deflection controls the optical beam spatially by using ultrasonic sound waves to diffract the laser beam depending on the acoustic frequency.
If we change the sound waveform, e.g. we gradually increase the frequency while maintaining the amplitude, meaning we create a focal point and then quickly move on to another by changing the frequency again. With this technique we can perform 3D random-access scanning where the laser moves almost instantaneously between many points within a region.
This method is very useful when we want to image neuronal activity at high temporal resolution in 3D and analyze how the brain computes in real-time.
Zsolt: We have also realized that random-access point scanning is not enough for in vivo biological applications, and that’s why we have developed special scanning techniques to be able to image not only points but extend these to planar or volume elements in 3D. Thanks to the work carried out by our scientists and engineers, it is possible to follow the 3D curvature of one or more dendrites with their spines at the same time.
Máté: The first step of the measurement is to select guiding points based on a z-stack, where the microscope scans several planes at different focal distances to build up an extended 3D image like a reference map.
The next step is to fit our 3D trajectories according to the z stack taken in advance and acquire images from only these points, rectangulars and elongated ribbons (Szalay et al. 2016 Neuron). This imaging technique allows you to see all of the components within your region of interest, including the cells bodies, dendrites and even the smaller protrusions.
For example one of our unique AO volume scanning method is the snake scanning, where we extend the pre-selected ribbons to a 3D structures (cuboids). This scanning option is very useful for imaging longer dendritic segments and dendritic spins which can be located in hidden and overlapping positions.
The FEMTO3D Atlas enables its users to rapidly and simultaneously scan both neuronal somata and subcellular domains in 3D with 3D random-access scanning method, up to a million times faster than classical scanning methods.
We continue to develop new scanning modes and recently released new options at SfN 2019. The new high-speed arbitrary frame mode of Atlas gives the option to acquire high-speed raster scanning of several cortical layers simultaneously or record individual neurons with their dendritic arborization which are not parallel with the front lens of the objective.
Femto3D Atlas - 3D dendritic imaging with parallel electrophysiology
Raster scanning on the FEMTO3D Atlas.
Why is it important to be able to gather data from thousands of neurons, simultaneously?
Máté: A single, tiny piece of a puzzle is also very interesting, for example, examine the function of a single ion channel is incredibly important. However, we also want to see the functionality of the brain in a living animal on a grander scale to understand the processes that lead to physiological functions and finally neurological diseases. To do this, you need an imaging technique that will allow you to visualize a high number of cells and record their activity with good spatial and temporal resolution.
The Femto3D Atlas is backed up by powerful software with a plethora of scanning modes. Which modules would you consider to be the most useful for interrogating neural networks, and why?
Máté: It is hard to choose the most useful, it always depends on the question you are trying to answer! Let me give you a couple of examples:
In my own research, where we are interested to understand cellular networks and network dynamics in the visual cortex of mice, I typically use the chessboard scanning mode. Where chessboard-like pattern contains the pre-selected neurons and their surrounding areas in each square. With this technique hundreds of neurons can be measured simultaneously in a near cubic millimeter volume and, importantly, motion correction can also be carried out to eliminate artifacts caused by tissue movement.
However, this can give us any number of cells, from 1 to 300! Beyond a certain number of cells, you’re sacrificing spatial and temporal resolution, so in my experiments, I usually select around 100-120 cells (with 15-20 Hz temporal resolution).
I also regularly use multi-cube scanning. In my experiments, I use this scanning mode to get a detailed 3D anatomical picture of the selected cells which helps to find the same cell ensemble day by day.
For dendritic imaging, or spine imaging, I would go with ribbon scanning (or in special cases, snake scanning – see above), in this way, it is possible to follow the 3D curvature of one or more dendrites with their spines at the same time. If you are interested in dendritic spine activity, you can also go for the multiple-line scanning.
Here, we extend scanning points along only a single dimension to perform measurements at a considerably higher speed on large number of spines simultaneously in 3D. Of course, this requires us to know the average trajectory of brain motion because we have to set the lines parallel with that.
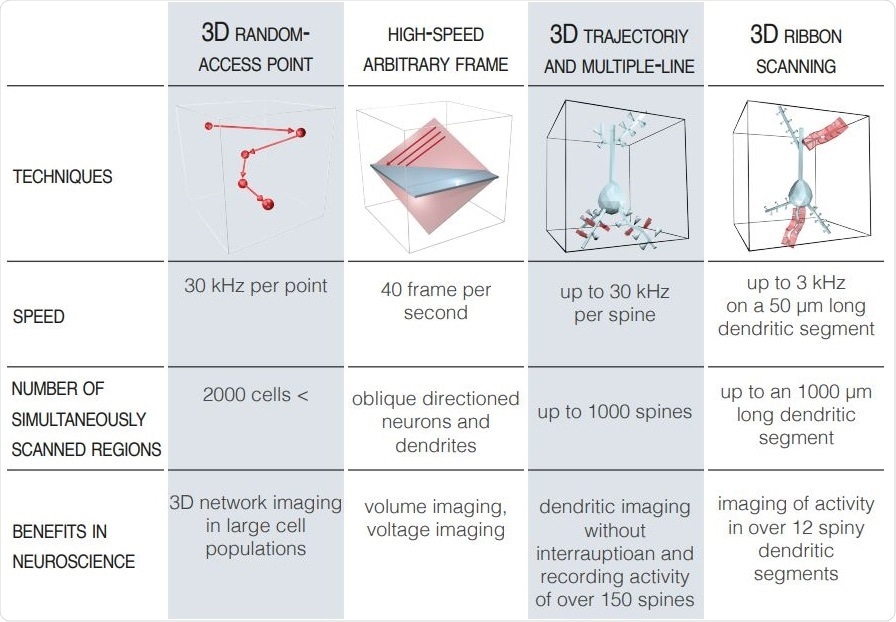 Advanced Scanning Modes. Image Credit: Femtonics.
Advanced Scanning Modes. Image Credit: Femtonics.
As advances continue to be made in the field of 3D imaging, how do you think the Femto3D Atlas will evolve over the next decade?
We sincerely believe that our innovation generally assists to the researchers acquiring even better and convincing results in their studies while the Femto3D ATLAS opens new horizons in their work. To make it happen, we want to ensure this great instrument getting available for every end-user in the neuroscience community.
Gergely Katona, CEO of Femtonics
Where can our readers find more information?
About Dr. Máté Marosi
 Máté is an Application Specialist and Senior scientist at Femtonics with a PhD degree of neuroscience and a strong academic background. He uses Femto3D ATLAS microscopes every day for his experiments.
Máté is an Application Specialist and Senior scientist at Femtonics with a PhD degree of neuroscience and a strong academic background. He uses Femto3D ATLAS microscopes every day for his experiments.
About Dr. Zsolt Iván
 Zsolt is the Head of Sales and Marketing at Femtonics. Zsolt Graduated in specialized pharmacology, clinical studies and economy. He joined Femtonics 3 years ago and worked with the Femto3D ATLAS since the beginning of development.
Zsolt is the Head of Sales and Marketing at Femtonics. Zsolt Graduated in specialized pharmacology, clinical studies and economy. He joined Femtonics 3 years ago and worked with the Femto3D ATLAS since the beginning of development.
About Femtonics
Femtonics was founded in 2005 by two scientists as a spin-off R&D company with roots in the Institute of Experimental Medicine of the Hungarian Academy of Sciences. Since then, we have expanded into a multidisciplinary team and became one of the most innovative manufacturers of two-photon laser scanning microscopes. Our microscopes provide the most innovative technologies, fitting a wide variety of in vivo and in vitro biological applications.