A study led by researchers at the University of Southampton, UK, has identified a novel short isoform of the full-length receptor that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses to gain entry to host cells.
The short isoform of angiotensin-converting enzyme 2 (ACE2), which the authors have called “short ACE2,” is expressed in the airway epithelium – the main infection site for SARS-CoV-2.
Lead authors Cornelia Blume, Claire L Jackson, and colleagues say that the short ACE2 is upregulated in response to stimulation with interferon (IFN)-beta and rhinovirus infection, but not SARS-CoV-2 infection.
They also report that the binding site for the viral spike protein that SARS-CoV-2 requires for attachment to long ACE2 is absent on short ACE2 and that overall, the findings suggest this short isoform may play a role in host susceptibility to infection.
The researchers say the findings may have significant implications for the development of treatments for COVID-19.
A pre-print version of the paper is available on the server bioRxiv*, while the article undergoes peer review.
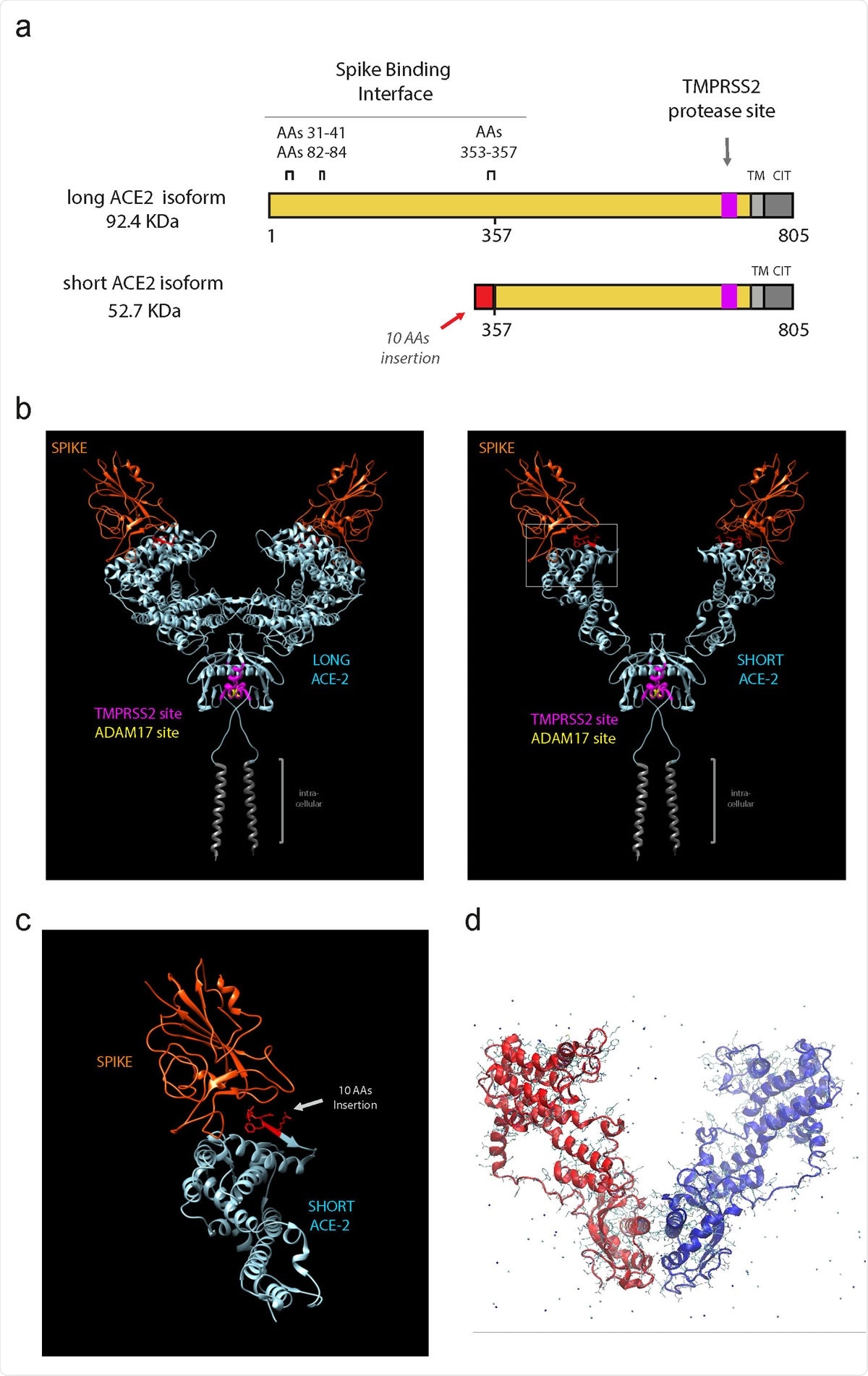
Short ACE2 lacks most of Spike binding regions but contains TMPRSS2 and ADAM17 proteases site

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
ACE2 protects against lung injury, although it is not clear how
Since the COVID-19 outbreak first began in Wuhan, China, late last year, SARS-CoV-2 has spread at an unprecedented rate, infecting approximately 18 million people and causing around 690,000 deaths.
Researchers around the world are racing to understand the underlying mechanisms of infection and disease to help identify the factors that influence susceptibility and potential therapeutic strategies.
ACE2 is a transmembrane carboxypeptidase with several well-known functions. The key substrate of ACE2 is angiotensin II, an important component in the renin-angiotensin system, which regulates blood pressure. ACE2 is also involved in glucose homeostasis and pancreatic beta-cell function.
“Interestingly, ACE2 plays an important role in protection from acute lung injury,” writes the team.
Animal studies have shown that the expression of ACE2 is downregulated in acute lung injury and that ACE2 activation can protect against pulmonary hypertension.
“Although the molecular mechanism by which ACE2 protects against acute lung injury remains unclear, it is known that the carboxypeptidase function of ACE2 is required to confer this protection,” say the researchers.
The expression of ACE2 in human nasal epithelia and lung tissue is controlled by interferon-responsive promoters, and the activation of interferon-responsive genes is key to defending against viral infection. Studies have shown that exposure to both IFN and the influenza virus increases ACE2 expression in the airway.
It has also been shown that multiciliated cells appear to be one of the main targets of SARS-CoV-2 infection, suggesting that cilia play an essential role, say Mennella and colleagues.
However, despite the important role that ACE2 plays in SARS-CoV-2 infection, researchers still do not fully understand its expression, including how this is influenced by viral infection.
The researchers identify a novel, short transcript of ACE2
Mennella and colleagues say that until now, the ACE2 gene had been thought to consist of 19 exons and to encode five transcripts, two of which encode the same ACE2 protein consisting of 805 amino acids.
However, the researchers have identified a novel, short 11-exon transcript of ACE2 that is translated into an ACE2 isoform made up of 459 amino acids.
The team found that unlike long ACE2, which is expressed in various tissues, this short ACE2 is only expressed in the airways, liver, and kidney.
Expression of short ACE2 was highest in the primary respiratory epithelia, particularly in the nasal epithelium, where it was expressed at higher levels than long ACE2.
In primary airway cells, short ACE2 was also upregulated in response to treatment with IFN-beta treatment and infection with respiratory rhinovirus, but not infection with SARS-CoV-2.
“We further show that the short transcript is an interferon-regulated gene and is more strongly induced by IFN-beta and viral infection than long ACE2,” writes the team.
The short isoform may be involved in regulating amino acid transport in the airway
Modeling studies suggested that short ACE2 retains the transmembrane domain and collectrin domain of long ACE2, indicating that the short isoform may be involved in regulating amino acid transport in the airway, say Mennella and colleagues.
“The short form also retains the catalytic residues conferring carboxypeptidase function, suggesting that the short isoform may retain some catalytic activity,” the team adds.
The researchers also report that most of the key residues needed for binding of the spike protein are not present on the short isoform, and they do not think short ACE2 is a viral entry point for SARS-CoV-2.
Mennella and colleagues say that although the function of short ACE2 is not yet clear, the data clearly show that this isoform is expressed in the airways, particularly in the ciliated cells of the nasal and bronchial epithelium.
“We hypothesize that this short isoform of ACE2 plays an important physiological role in the airway and, in addition, that it may influence host susceptibility to SARS-CoV-2 infection,” they write.
The researchers say the findings could have significant consequences for the development of treatments designed to tackle COVID-19.
“Our discovery also has implications for the numerous studies reporting on ACE2 expression levels and differences in levels of expression along airways, across age groups and disease groups, including COVID-19 disease severity,” they add.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
A novel isoform of ACE2 is expressed in human nasal and bronchial respiratory epithelia and is upregulated in response to RNA respiratory virus infection, Cornelia Blume, Claire L Jackson, Cosma, Mirella Spalluto, Jelmer Legebeke, Liliya Nazlamova, Franco Conforti, Jeanne-Marie Perotin-Collard, Martin Frank, Max Crispin, Janice Coles, James Thompson, Robert A Ridley, Lareb S N Dean, Matthew Loxham, Adnan Azim, Kamran Tariq, David Johnston, Paul J Skipp, Ratko Djukanovic, Diana Baralle, Chris McCormick, Donna E Davies, Jane S Lucas, Gabrielle Wheway, Vito Mennella, bioRxiv 2020.07.31.230870; doi: https://doi.org/10.1101/2020.07.31.230870, https://doi.org/10.1101/2020.07.31.230870
- Peer reviewed and published scientific report.
Blume, Cornelia, Claire L. Jackson, Cosma Mirella Spalluto, Jelmer Legebeke, Liliya Nazlamova, Franco Conforti, Jeanne-Marie Perotin, et al. 2021. “A Novel ACE2 Isoform Is Expressed in Human Respiratory Epithelia and Is Upregulated in Response to Interferons and RNA Respiratory Virus Infection.” Nature Genetics 53 (2): 205–14. https://doi.org/10.1038/s41588-020-00759-x. https://www.nature.com/articles/s41588-020-00759-x.