The nanoscale organization is critical in immune response programming, and virus-like particles (VLPs) improve immune responses by boosting B-cell receptor clustering and lymph node uptake. Current vaccination regimens have limited vaccine protection, new pathogen variations, and questionable treatment effectiveness. VLPs containing numerous protein variations are promising for broadening immune responses, possibly defending against strains and new viruses such as SARS-CoV-2. Recent techniques use stochastically ordered protein variations to promote B cell growth.
Summary of timeline and antigens for this set of immunizations. Study: Proactive vaccination using multiviral Quartet Nanocages to elicit broad anti-coronavirus responses
About the study
In the present study, researchers devised a proactive vaccination technique based on multiple-viral Quartet nanocages. These nanocages can combat various coronaviruses by evoking broad antiviral responses.
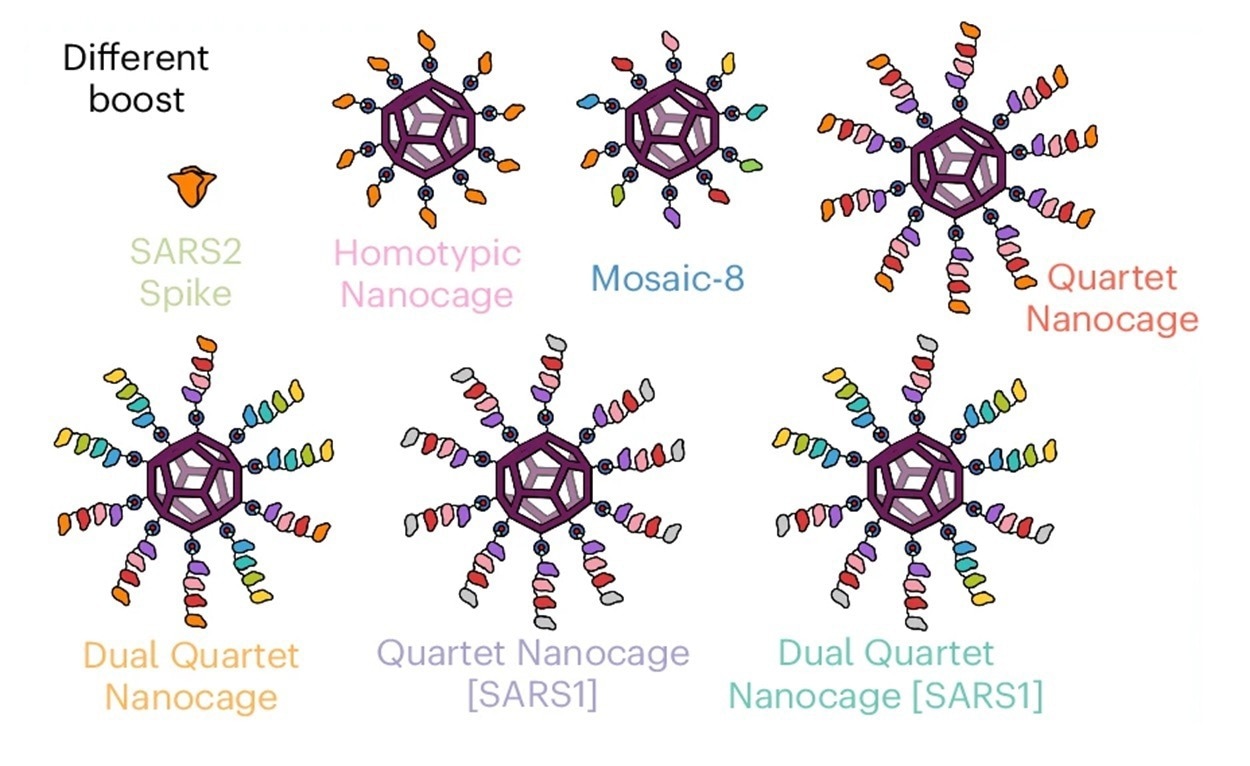
The multiple-viral quartet vaccine comprised RBDs from four viruses linked together to form a polypeptide chain. The researchers constructed the antigenic quartets using a terminal SpyTag to originate from SpyCatcher003-mi3 nanocages, resulting in proteinaceous nanoparticles with branched structures. They examined immune responses to various sarbecoviruses exhibited on Quartet Nanocages, sarbecoviruses not found in the chain, and SARS-CoV-2 variants of concern (VOCs). They also genetically combined RBDs from the evolutionarily similar sarbecoviruses SHC014, Rs4081, RaTG13, and SARS-CoV-2 Wuhan Hu-1 strain to create a multi-viral quartet.
The team used a plug-and-display vaccine assembly of mosaic and Quartet nanocages to effectively multimerize single or Quartet RBDs connected to SpyTag via spontaneous isopeptide bond formation. They also created the Alternate Multiviral Quartet, which includes SpyTag followed by RBDs from various sarbecoviruses: pang17, RmYN02, Rf1, and WIV1. To study the association between chain location and immunogenicity, they evaluated SARS-CoV-2 Wuhan or Delta variant neutralization with a tenfold larger antigen dosage and the squalene-based adjuvant AddaVax to improve neutralization.
To evaluate reactions to RBDs at various distances from SpyCatcher003-mi3, the researchers used enzyme-linked immunosorbent assays (ELISA) on Quartet antigens and a panel of anti-SARS-CoV-2 monoclonal antibodies. They also created a quartet without flexible Gly-Ser linkers to separate the various RBDs and tested the No-Linker Quartet Nanocage against the typical Quartet Nanocage.
The team used polymerase chain reaction (PCR) to clone RBD constructs, which were confirmed using Sanger sequencing. Quartet RBD constructs were cloned using Gibson assembly and then converted into E. coli BL21 (DE3) cells for bacterial expression and cell culture studies. SpySwitch was used to purify RBDs, Quartets, and SpyTag-MBP, which the researchers evaluated using SDS-PAGE electrophoresis. They isolated SARS-CoV-2 spike (S) proteins by Ni-NTA affinity chromatography, PNGase F digestion, and transmission electron microscopy (TEM). They extracted endotoxins from vaccine components and used bicinchoninic acid tests to determine their concentrations.
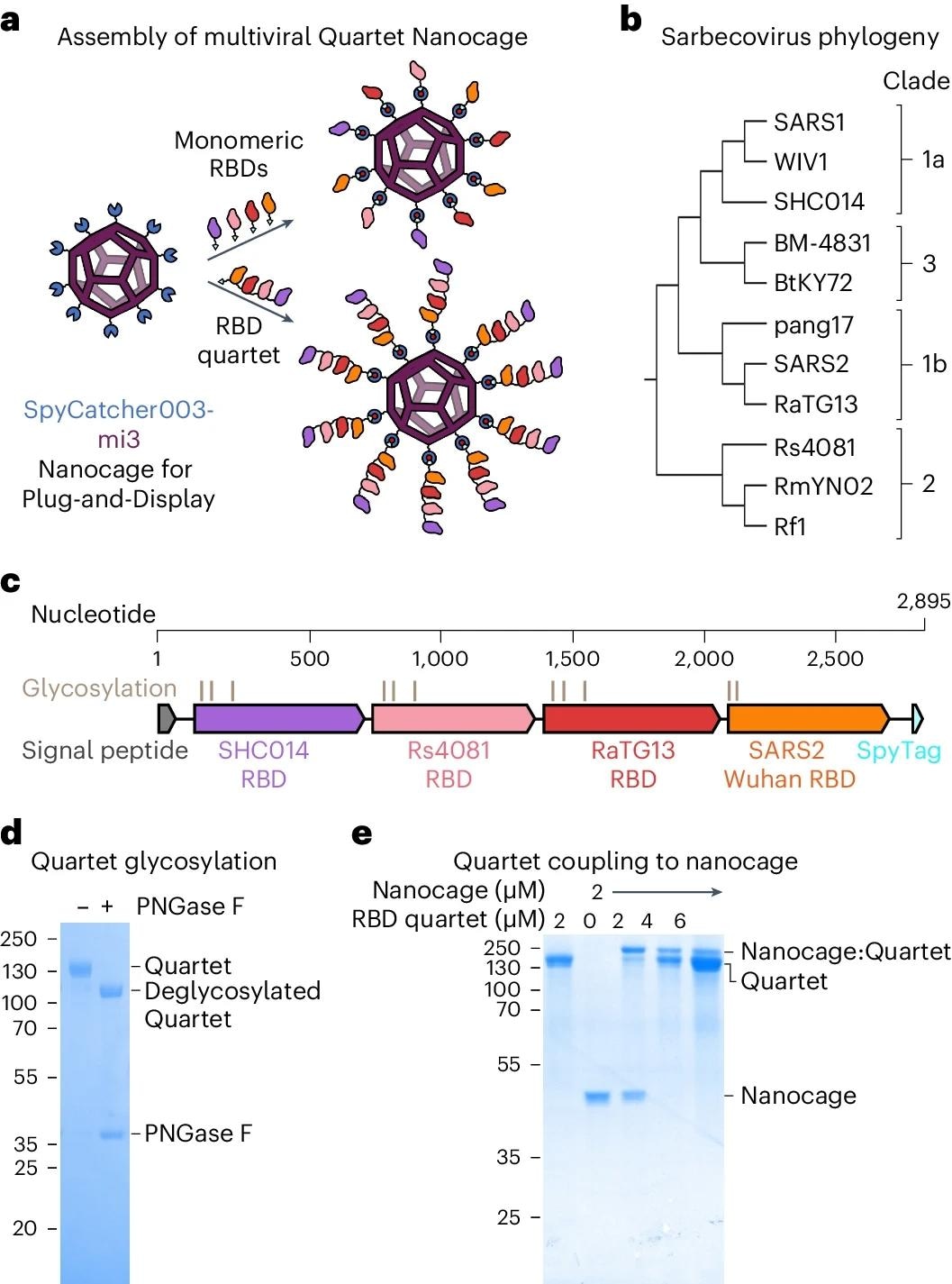
a, Plug-and-Display vaccine assembly of mosaic and Quartet Nanocages. Genetic fusion of SpyCatcher003 (dark blue) with mi3 (purple) allows efficient multimerization of single or Quartet RBDs linked to SpyTag (cyan) through spontaneous isopeptide bond formation (marked in red). Only some antigens are shown in the schematic for clarity. b, Phylogenetic tree of sarbecoviruses used in this study, based on RBD sequence. c, Genetic organization of the multiviral Quartet-SpyTag, indicating the viral origin of RBDs, N-linked glycosylation sites and tag location. d, Analysis of Quartet-SpyTag with SDS–PAGE/Coomassie staining, with or without PNGase F deglycosylation. A representative gel from two independent experiments. Molecular weight markers are in kDa. e, Coupling of RBD Quartet to SpyCatcher003-mi3 Nanocage at different molar Nanocage:antigen ratios, analysed by SDS–PAGE/Coomassie. A representative gel from two independent experiments. Molecular weight markers are in kDa.
Results
Quartet nanocages, branching nanoparticles with a branched architecture, have been shown to stimulate significant levels of neutralizing antibody titers against various coronaviruses, even ones not included in the vaccination. Antibodies responded similarly to RBDs at the nanocage or their branch tip. Among mice primed with SARS-CoV-2 S, booster vaccinations using Quartet nanocages improve the breadth and intensity of the otherwise limited immune response. The Quartet Nanocage, comprising Omicron XBB.1.5 'Kraken' RBD, elicited neutralizing antibodies bound to several sarbecoviruses. The size and breadth of antibody induction indicate that Quartet Nanocages can generate neutralizing antibodies against several viruses, preparing for new epidemic disease risks.
Homotypic Nanocage and uncoupled receptor-binding domain vaccination produced modest responses against sarbecovirus receptor-binding domains, with the Homotypic Nanocage eliciting the most robust cross-reactive responses against RaTG13 receptor-binding domains. However, vaccination with uncoupled quartets and quartet nanocages produced significant responses to all investigated RBDs, including high heterotypic responses to SARS-CoV-1 and BM-4831 RBDs.
The Dual Quartet Nanocage, featuring the same RBDs as Mosaic-8, was created by combining the Quartet and its alternate with SpyCatcher003-mi3. Quartet Nanocage and the dual-quartet type produced the highest post-boost antibody titers, whereas Quartet Nanocage and Mosaic-8 elicited similar, moderately robust anti-SARS-CoV-1 responses.
The Quartet and Mosaic immunogens exhibited higher antibody binding to zoonotic coronaviruses, while XBB.1.5 immunogens provided higher SARS2 XBB.1.5 pseudovirus neutralization, indicating that the Quartet Nanocage could guard against antibody-escape variants and elicit widespread anti-arbovirus responses without SARS-CoV-2. Quartet nanocage without SARS-CoV-2 generated anti-SARS-CoV-2 antibodies and promoted responses to sarbecoviruses.
The study findings highlight the creation of improved vaccine platforms like Quartet Nanocages, which offer heterotypic protection against emerging zoonotic illnesses and enable proactive pandemic protection. These nanocages stimulate neutralizing antibodies against viral antigens. The solubility, thermostability, flexibility, and sequence divergence of sarbecovirus RBDs facilitated the efficient generation of different Quartets. The vaccination candidates demonstrated breadth comparable to or greater than Mosaic-8, with Omicron variants effectively avoiding neutralizing antibodies.