Numerous therapeutic advantages are offered by sustained-release drugs, which release active pharmaceutical ingredients (APIs) in a gradual manner. However, the QC of such drugs through conventional dissolution testing is time consuming. A rapid and simple alternative to conventional testing is near-infrared spectroscopy (NIRS), which enables fast and accurate determination of APIs released from sustained-release tablets when used in conjunction with suitable calibration models.
The so-called plasma peak phenomenon occurs after the consumption of a tablet, and at this point, the entire amount of the drug is released rapidly, causing a temporary peak increase of the concentration of the API in the blood plasma of the patient.
Significant side effects are caused by this high API concentration based on the type of drugs. Moreover, for a lasting effect, drugs need to be frequently administered because the high plasma level is temporary and tends to dip once the active ingredient undergoes metabolism.
Reducing Side Effects with Sustained Release
As seen in Figure 1, sustained-release drug formulations can address the aforementioned problems faced with conventional drugs formulations by preventing the plasma peak phenomenon so that the plasma level can be reasonably sustained for a longer period.
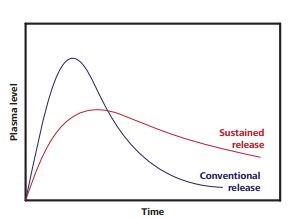
Figure 1. Conventional tablets (purple curve) release their active ingredients all at once. Meanwhile, sustained-release formulations (red) offer a gradual release.
Sustained-release formulations are particularly used for blood pressure regulation and hormone preparations, and as painkillers and antidepressants. Sustained-release tablets can be produced by a number of methods. In one method, tablets are coated to prevent their rapid decomposition in gastric juices. Special polymers are also present in most of the tablets to generate a matrix structure that enables slow and controlled release of APIs.
In tablets containing special polymers, the release time of the active ingredient content can be controlled by varying the concentration of polymers and plasticizers, if used. Ethyl cellulose and Eudragit NE 30 D are examples of the polymers commonly used in sustained-release formulations. Ethyl cellulose is often used in conjunction with the plasticizer, plasticizer acetyl tributyl citrate or ATBC.
Time-Intensive Tests
The main limitation of sustained-release formulations is the tedious, time-intensive QC procedures. Dissolution testing is the commonly followed QC procedure, wherein the tablet is dissolved into a solvent having similar properties of gastric juice. The determination of the free active ingredient is carried out at regular intervals over the release period of interest, typically 24 hours.
NIRS for Fast and Accurate Quality Control
In contrast to the dissolution method discussed above, NIRS used in conjunction with suitable calibration model enables accurate and fast prediction of the dissolution profile of tablets within a few minutes. It is a non-destructive testing method, thus facilitating the analysis of large quantities of the sample.
NIRS Analysis of Theophylline Tablets
The 2009 publication by Tabasi et al. described the NIRS analysis for evaluating the dissolution behavior of theophylline tablet, which are sustained-release formulations used in the treatment of asthma.
Tabasi and her team investigated the tablets that contained varying concentrations of the Eudragit NE 30 D polymer, which is responsible for decelerating the rate of release of the API. First, the NIR spectra obtained from several tablets with varying polymer content (0%, 5%, 10%, 15% and 20%) were recorded, and then the release time of the active ingredient was determined through the dissolution test (Figure 2).
Using the resulting data, the researchers created a calibration model for different measuring times: 1, 2, 3 and 4 hours. A correlation between the changes related to polymer content in the NIR spectrum and the values measured by dissolution tests was drawn based on the calibration models.
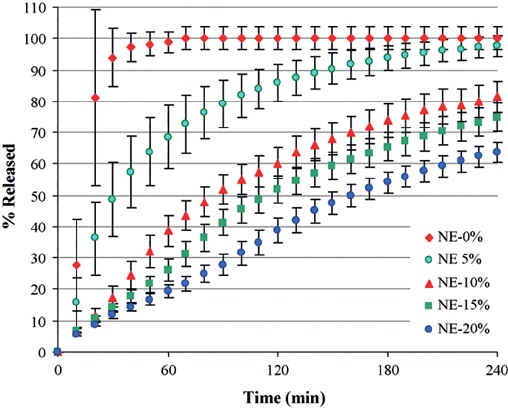
Figure 2. Active ingredient release from theophylline tablets containing different concentrations of the polymer Eudragit NE 30 D, as determined in a dissolution test.
Using Multivariate Analysis to Produce a Model
A spectrometer from Metrohm NIRS systems (Figure 3) is used for recording the NIR spectrum by irradiating the sample using light of varying wavelengths in succession. The process involved scanning of a specific wavelength range the NIR region and measuring either the transmittance or the reflectance based on the properties of the sample.

Figure 3. The Metrohm NIRS system
The measured value of transmittance or reflectance is plotted against the wavelength to obtain the spectrum. Multivariate analysis can be conducted through the representation of a sequence of spectra
in the matrix format, where n different spectra is obtained from a series of samples, with p measured values at p different wavelengths for each sample. Consequently, the matrix’ form is as follows:
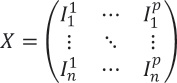
For the theophylline tablets, each row is a representation of a specific polymer concentration and consists of the reflectance values for every wavelength, averaged over various samples and measurements.
Modeling with PLS and PCA
A new orthogonal coordinate system is used for projecting the components of the data matrix in order to produce a calibration model using the partial least squares (PLS) regression. By choosing the orthogonal coordinate system, data representation can be done with the least number of components possible.
This approach looks like the principal component analysis (PCA) method. However, in the PLS method, both the NIRS data and the dissolution test values are used for defining a new coordinate system so that the covariance can be optimized, and consequently, the correlation between the NIRS data and the dissolution test data.
The model produced by the PLS method is able to predict the dissolution characteristics of the sample based on its NIR spectrum. Once the models are validated, they can be used for predicting the dissolution behavior of samples that are similar to selected samples. The comparison of measurements and predictions conducted by Tabesi and team for few select samples from the validation set is shown in Figure 4.
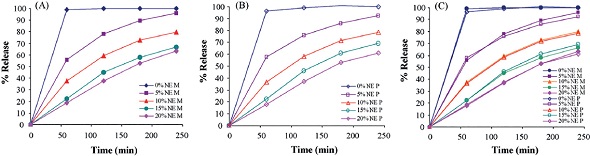
Figure 4. A Measured and B predicted dissolution behavior of selected samples. C shows the measured and predicted values superimposed on one another.
Conclusion
The dissolution behavior of tablets can be predicted easily and rapidly by NIRS. With the help of an appropriate calibration model for the tablet under analysis, NIRS is able to complete the dissolution behavior prediction within a few minutes. However, the accuracy of prediction depends on the chosen model.
The prediction results can be accurate and reliable when a thoroughly validated model is used. The NIRS method brings down the time and effort spent on the QC of tablets drastically. Furthermore, the non-destructive nature, ease of operation and the speed of NIRS enables the analysis of comparatively larger sample quantities as opposed to dissolution testing.
References
- Tabasi, S. H. et al. (2009) Int. J. Pharm. 382, 1–6
About Metrohm
At Metrohm is one of the world’s most trusted manufacturers of high-precision instruments for chemical analysis. Metrohm was founded in 1943 by engineer Bertold Suhner in Herisau, Switzerland. Today, Metrohm is represented in 120 countries by subsidiaries and exclusive distributors. The global Metrohm Group also includes the Dutch companies Metrohm Applikon and Metrohm Autolab, manufacturers of online analyzers and instruments for electrochemical research, respectively. Recently, the Metrohm Group was joined by Metrohm Raman, a leading manufacturer of handheld Raman spectrometers.
Metrohm is the global market leader in analytical instruments for titration. Instruments for ion chromatography, voltammetry, conductivity, and stability measurement make the Metrohm portfolio for ion analysis complete. Instruments for Near-infrared and Raman spectroscopy are another, strongly growing segment of the Metrohm portfolio.
Metrohm is a problem solver, both in the laboratory and within the industrial process. To this end, the company offers their customers complete solutions, including dedicated analytical instrumentation as well as comprehensive application know-how. More than 30% of the company’s employees at the Metrohm international headquarters in Herisau work in R&D.
Metrohm has been owned 100% by the non-profit Metrohm Foundation since 1982. The Metrohm Foundation, which does not exert any influence on the company’s business operations, sponsors gifted students in the natural sciences, supports charitable and philanthropic purposes and, above all, ensures the independence of the company.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.