Chimeric antigen receptor (CAR) therapies utilize engineered receptors that are able to redirect immune cells to identify and destroy cancer cells that express a specific antigen. At present, CAR-T cells (T cells equipped with CAR) dominate the CAR therapy field.
More than 700 clinical trials have been registered since 2017 that involve CAR-T therapy. The U.S. Food and Drug Administration (FDA) has given approval for six CAR-T therapies for treating lymphomas, leukemia, and multiple myeloma (see Figure 1).
Despite the clinical significance of CAR-T therapy, there are aspects of the therapy that cause limitations. Firstly, collecting T cells is time-consuming and costly, which means this may not be an option for patients with a compromised immune system.
Secondly, CAR-T therapies mostly concentrate on the treatment of hematological malignancies and have a limited effect on the treatment of solid tumors. Thirdly, CAR-T cells do not easily penetrate the tumor microenvironment (TME), which can negatively impact their therapeutic function.
To address these limitations, there are promising CAR designs emerging for multi-target CAR-T in addition to novel therapies that utilize other immune cell types such as CAR-Macrophage (M) and CAR-Natural killer (NK) cells.
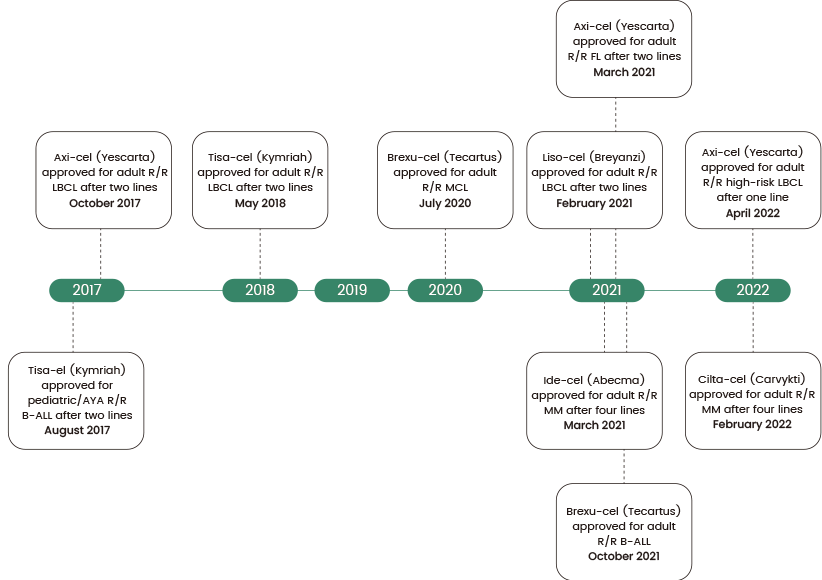
Figure 1. Timeline of CAR-T FDA approvals in the US. B-ALL, B cell acute lymphoblastic leukemia; LBCL, large B cell lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; R/R, relapsed/refractory. Source: https://doi.org/10.1136/bmj-2021-068956
CAR molecule design
CAR is a modified fusion protein developed from the T cell receptor containing an antigen binding domain and various intracellular signaling domains. The CAR molecule is critical for therapeutic success because it targets CAR-T cells to destroy cancer cells.
To be effective, the CAR molecule must have several essential features, such as high specificity for the target antigen to ensure that CAR-T cells only attack cancer cells and not healthy ones.
The CAR molecule should also efficiently activate CAR-T cells to invoke a robust immune response and reduce off-target impacts to minimize the risk of unintended harm to healthy tissues.
As a result, extensive research efforts have concentrated on the optimization of the CAR molecule's specificity, efficiency, and safety to make CAR-T cell therapy a more reliable and effective option for cancer treatment.
CARs consist of three key components: an extracellular domain, an intracellular signaling domain, and a transmembrane domain. Included in the extracellular domain are the signal peptide and the antigen-recognition domain, comprised of a single-chain fragment variant (scFV).
Typically, the transmembrane domain is an alpha helix that spans the cell membrane and is vital for sustaining the receptor's surface expression and stability. The intracellular domain experiences conformational changes upon antigen binding to recruit and phosphorylate downstream signaling proteins.
Though it may include several functional units, the T cell co-receptor CD3ζ's intracellular domain is the main component for the majority of CARs. This comprises three immunoreceptor tyrosine-based activation motifs (ITAMs), which are vital for signal transduction (see Figure 2a).
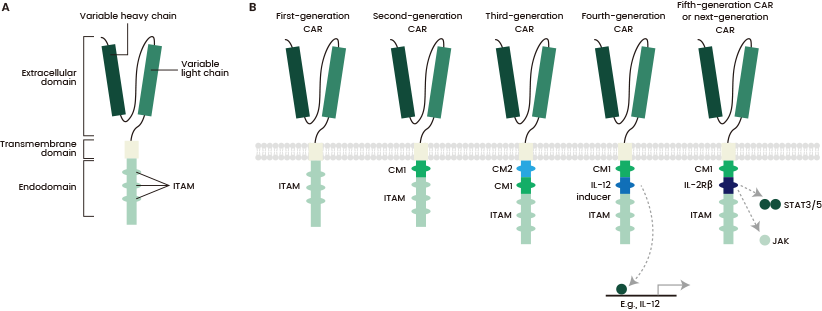
Figure 2. Structure of different chimeric antigen receptor (CAR) generations. Source: https://doi.org/10.1038/s41416-018-0325-1
There are five generations of CARs with different compositions and structures.
The first generation consisted of only a CD3ζ intracellular domain, which caused low cytotoxicity and proliferation. The second generation included the addition of a co-stimulatory domain to improve T cell cytotoxicity and proliferation, while the third added a co-stimulatory domain.
The fourth generation, which is known as TRUCKs, involved a protein constitutively or inducibly expressed upon CAR activation, for example, IL-12.
At present, the fifth generation is under development. It includes a truncated cytoplasmic IL-2 receptor β-chain domain with a binding site for the transcription factor STAT3, supplying all three synergistic signals necessary for full T cell activation and proliferation (see Figure 2b).
CAR-Natural Killer (NK)
Natural killer (NK) cells are innate lymphoid cells that have the ability to detect and eradicate tumor and virus-infected cells. Over the recent two decades, CAR-NK cells have been applied successfully as immunotherapy for advanced-stage leukemia.
Novel strategies to improve NK cell potency and persistence have been developed with the utilization of checkpoint inhibition, co-stimulatory signaling, and cytokine armors, which redirect NK cell specificity to the tumor via CAR or engager molecules.
The results of the first generation of CAR-NK cell therapies demonstrated great promise, and this encouraged continued innovation in the field, such as the creation of genetically modified CAR-NK cells, which have the ability to destroy tumor cells both in CAR-independent and CAR-dependent ways.
Compared to conventional CAR-T cells, both unmodified NK cells and CAR-NK cells possess safety advantages, including decreased risk of graft-versus-host disease and alloreactivity.
As a result, they are perfect for immune cell sourcing from sources other than autologous cells, including peripheral blood mononuclear cells (PBMCs), induced pluripotent stem cells (iPSCs), NK92 cells, and umbilical cord blood (UCB).
NK cells produce different cytokines compared to T cells, such as IL-3, GM-CSF, and TNF-α, reducing the incidence and severity of inflammatory cytokine release syndrome and neurological toxicity. The limited lifespan of CAR-NK cells also decreases the risks of cell toxicity.
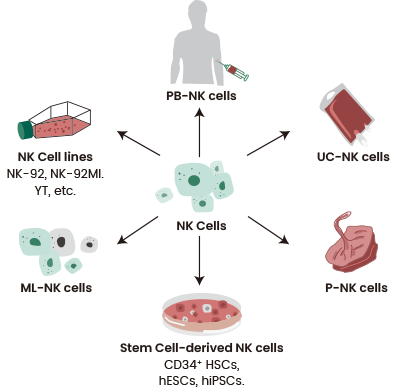
Figure 3. Various sources of NK cells. Source: https://doi.org/10.1186/s40364-022-00364-6
According to clinicaltrials.gov, 26 clinical trials are currently being conducted on CAR-NK targeting specific antigens. Examples of these include:
- Acute myeloid leukemia (CD123 and NKG2D)
- Multiple myeloma (CLL1, CD33, and BCMA)
- Solid tumors (PD1, Lag3, CTLA4, and 5T4)
- Relapsed B cell non-Hodgkin lymphoma (CD70 and CD19)
- Prostate cancer (PSMA)
- Small cell lung cancer (DLL3)
- Head and neck cancer (PD-L1)
However, CAR-NK therapies are not yet approved by the FDA.
CAR-NK and CAR-T therapies share common challenges, such as on-target/off-tumor toxicity, where the CAR-modified cells attack the tumor and healthy tissues expressing the targeted antigen.
Another example is the potential for antigen loss or downregulation, which leads to treatment failure.
The immunosuppressive TME causes difficulties for both therapies in targeting solid tumors. However, CAR-NK has various advantages over CAR-T because of its potential for off-the-shelf use, ability to target a broader range of tumors (as a result of major histocompatibility complex restrictions), and lower risk of cytokine release syndrome (CRS).
Nevertheless, CAR-NKs demonstrate lower potency and persistence than CAR-T cells, which could restrict their efficacy against certain cancers.
Although the half-life of NK cells is briefer and can decrease the risk of on-target/off-tumor toxicity to normal cells, it can also add to poor cytotoxicity in an immunosuppressive TME.
Producing sufficient NK cells is necessary for adoptive immunotherapy and cytokines such as IL-2, IL-7, IL-12, IL-15, and IL-21 are essential for in vivo growth and survival of NK cells.
Sino Biological has successfully created high-activity, high-purity GMP-grade cytokines to help the development of human adoptive immunotherapies.
CAR-M
Contrastingly to T and NK cells, macrophages can infiltrate and persist within tumors. They also display a distinct mechanism of tumor cell killing: phagocytosis. CAR-M and the more recent CAR-M derived from induced pluripotent stem cells (CAR-iMac) are promising alternatives to traditional CAR-T therapy.
The central components of CAR-M are comparable to CAR-T, including an extracellular domain that delivers specific recognition via a single-chain variable fragment (scFv), such as HER2 or CD19, a hinge domain, a transmembrane domain (mostly CD8), and an intracellular domain for dedicated downstream signaling, such as FcgR and CD3z (see Figure 4).
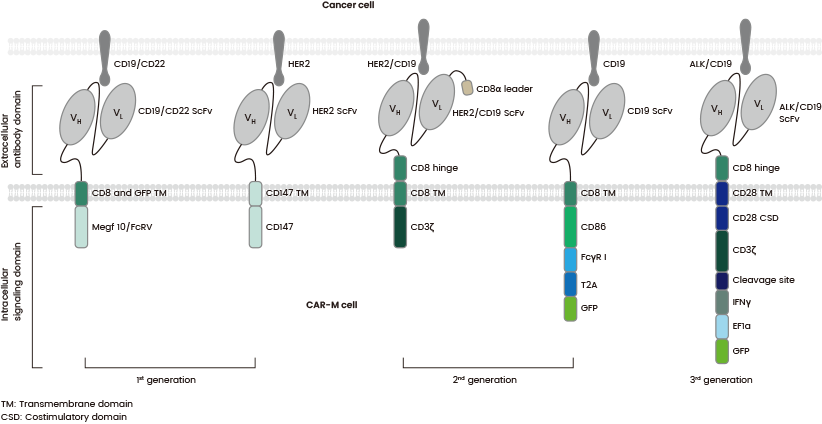
Figure 4. The structures of various generations of CAR-Ms which differ in their intracellular domain. Source: https://doi.org/10.1016/j.ebiom.2022.103873
Like other CAR-based immunotherapies, CAR-M encounters cytokine toxicity and antigen loss challenges. However, CAR-M has various advantages over both CAR-NK and CAR-T, including TME infiltration, superior immune cell trafficking, and the ability to overcome immunosuppressive TMEs.
Tumor-associated macrophages (TAMs) are especially abundant in tumors and have the ability to migrate efficiently to tumor sites.
Studies have shown that hypoxia-induced tumor cells and stroma produce chemokines such as CCL12, CCL2, M-CSF, and growth factor VEGFA which correlates with macrophage accumulation.
However, CAR-M therapy remains a new approach, and its research is largely preclinical. A key challenge of macrophage-based therapies is the balance between therapeutic efficacy and macrophage expansion.
Although iPSCs could serve to generate macrophages, offering a potential solution for efficient macrophage production, this approach is still under development.
Future outlook
There has been groundbreaking research within the field of CAR-T therapy, and it is the cell therapy with the greatest promise for hematological tumors, as evidenced by several FDA approvals.
Ongoing research intends to overcome the limitations associated with CAR-T therapy and investigate alternative CAR-based therapies such as CAR-M and CAR-NK.
Although new strategies for B cells and dendritic cells have been introduced, their therapeutic potential demands further investigation. Innovative approaches, including the development of multi-target CAR-Ts, have surfaced to expand antigen coverage, with recent strategies targeting ≥2 antigens.
Multi-target CAR-T cells have demonstrated an improvement in anti-tumor efficacy, CAR-T cell persistence and specificity, prevention of tumor escape, and decreasing the risk of severe CRS. Additionally, many studies utilizing dual-target combinations and even trivalent CAR-T cells are currently in progress.
However, the efficacy of novel CAR-based cell therapy continues to be limited by a lack of clinical experience, and multi-target CAR-T cells only address antigen loss. CAR-based cell therapies still require issues to be addressed, including cytotoxicity, effective cell generation, and tumor infiltration.
Sino Biological is a global leader in the supply of bioreagents and CRO services for biopharmaceutical research.
It provides comprehensive solutions for CAR-NK and CAR-T cell therapy development. Its reagents and services support clients through the full developmental process, from early target discovery to preclinical research and development.
References
- Maakaron, J., Hu, M., & Jurdi, N. E. (2022). Chimeric antigen receptor T cell therapy for cancer: clinical applications and practical considerations. BMJ, e068956. https://doi.org/10.1136/bmj-2021-068956
- Qin, V. M., D'Souza, C., Neeson, P. J., & Zhu, J. J. (2021). Chimeric Antigen Receptor beyond CAR-T Cells. Cancers, 13(3), 404. https://doi.org/10.3390/cancers13030404
- Pan, K., Farrukh, H., Chittepu, V. C. S. R., Xu, H., Pan, C. X., & Zhu, Z. (2022). CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. Journal of experimental & clinical cancer research: CR, 41(1), 119. https://doi.org/10.1186/s13046-022-02327-z
- Su, S., Lei, A., Wang, X., Lu, H., Wang, S., Yang, Y., Li, N., Zhang, Y., & Zhang, J. (2022). Induced CAR-Macrophages as a Novel Therapeutic Cell Type for Cancer Immune Cell Therapies. Cells, 11(10), 1652. https://doi.org/10.3390/cells11101652
- SafarzadehKozani, P., SafarzadehKozani, P., & O'Connor, R. S. (2021). In Like a Lamb; Out Like a Lion: Marching CAR T Cells Toward Enhanced Efficacy in B-ALL. Molecular cancer therapeutics, 20(7), 1223–1233. https://doi.org/10.1158/1535-7163.MCT-20-1089
- Barros, L. R. C., Couto, S. C. F., da Silva Santurio, D., Paixão, E. A., Cardoso, F., da Silva, V. J., Klinger, P., Ribeiro, P. D. A. C., Rós, F. A., Oliveira, T. G. M., Rego, E. M., Ramos, R. N., & Rocha, V. (2022). Systematic Review of Available CAR-T Cell Trials around the World. Cancers, 14(11), 2667. https://doi.org/10.3390/cancers14112667
- Laskowski, T., Biederstädt, A., &Rezvani, K. (2022). Natural killer cells in antitumour adoptive cell immunotherapy. Nature Reviews Cancer, 22(10), 557–575. https://doi.org/10.1038/s41568-022-00491-0
- Zhang, L., Meng, Y., Feng, X., & Han, Z. (2022). CAR-NK cells for cancer immunotherapy: from bench to bedside. Biomarker Research, 10(1). https://doi.org/10.1186/s40364-022-00364-6
About Sino Biological Inc.

Sino Biological is an international reagent supplier and service provider. The company specializes in recombinant protein production and antibody development. All of Sino Biological's products are independently developed and produced, including recombinant proteins, antibodies and cDNA clones. Sino Biological is the researchers' one-stop technical services shop for the advanced technology platforms they need to make advancements. In addition, Sino Biological offers pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates.
Sino Biological's core business
Sino Biological is committed to providing high-quality recombinant protein and antibody reagents and to being a one-stop technical services shop for life science researchers around the world. All of our products are independently developed and produced. In addition, we offer pharmaceutical companies and biotechnology firms pre-clinical production technology services for hundreds of monoclonal antibody drug candidates. Our product quality control indicators meet rigorous requirements for clinical use samples. It takes only a few weeks for us to produce 1 to 30 grams of purified monoclonal antibody from gene sequencing.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.