GTx’s scalable electroporation technology is clinically verified and can handle even the most demanding cellular engineering requirements, from product development to translation into clinical trials.
Highlights
- Rapidly develop with a known regulatory route backed by an FDA Master File
- Manufacture confidently with closed, cGMP-compliant, ISO-certified, and CE-marked products
- Move forward with 21 CFR Part 11-enabled software
- Enjoy the proprietary Flow Electroporation® technology from MaxCyte
- Transfect cells quickly from 75 thousand to 20 billion cells
Product features
- Integrated touch screen
- Effortless operation at the touch of a finger
- Enhanced software user interface
- Save time with updated software that offers more functionality and intuitive usage
- Reduced footprint
- Bench-scale, modular equipment
- LED status indicators
- With six vibrant and clearly defined status modes, users have immediate access to the instrument and run status
- Elegant design
- Experience a modern, sleek look that fits into any high-tech lab space
- Barcode reader
- Record crucial sample processing information and reduce manual data entry
- Network capable
- Produce and save run reports automatically onto a shared local disc
- Retractable bag hooks
- When processing high volumes, use convenient hooks and then fold them away when not in use
MaxCyte® offers more than just an instrument; the customer becomes a partner with a cell engineering specialist with the skills to assist users at every level of their research journey.

Image Credit: MaxCyte, Inc.
Compatible consumables and accessories
Supporting products
G-1000
With as few as 400 uL, up to 1 mL, and 2×106 to 2×108 cells, it is possible to clinically produce the therapeutic end product.
Features
- Made from inert, medical-grade materials to safeguard priceless cells
- Well designed for efficient loading and unloading of cells
- Improved design for enhanced handling
- Locking lid to secure sample

Image Credit: MaxCyte, Inc.
Closed buffer
Streamlined, sterile buffer exchange for automated cell concentration saves time.
Features
- 500 mL and 1 L bag options
- Compatible with closed process
- Connection flexibility
- Adaptable to cell concentration and buffer exchange processes
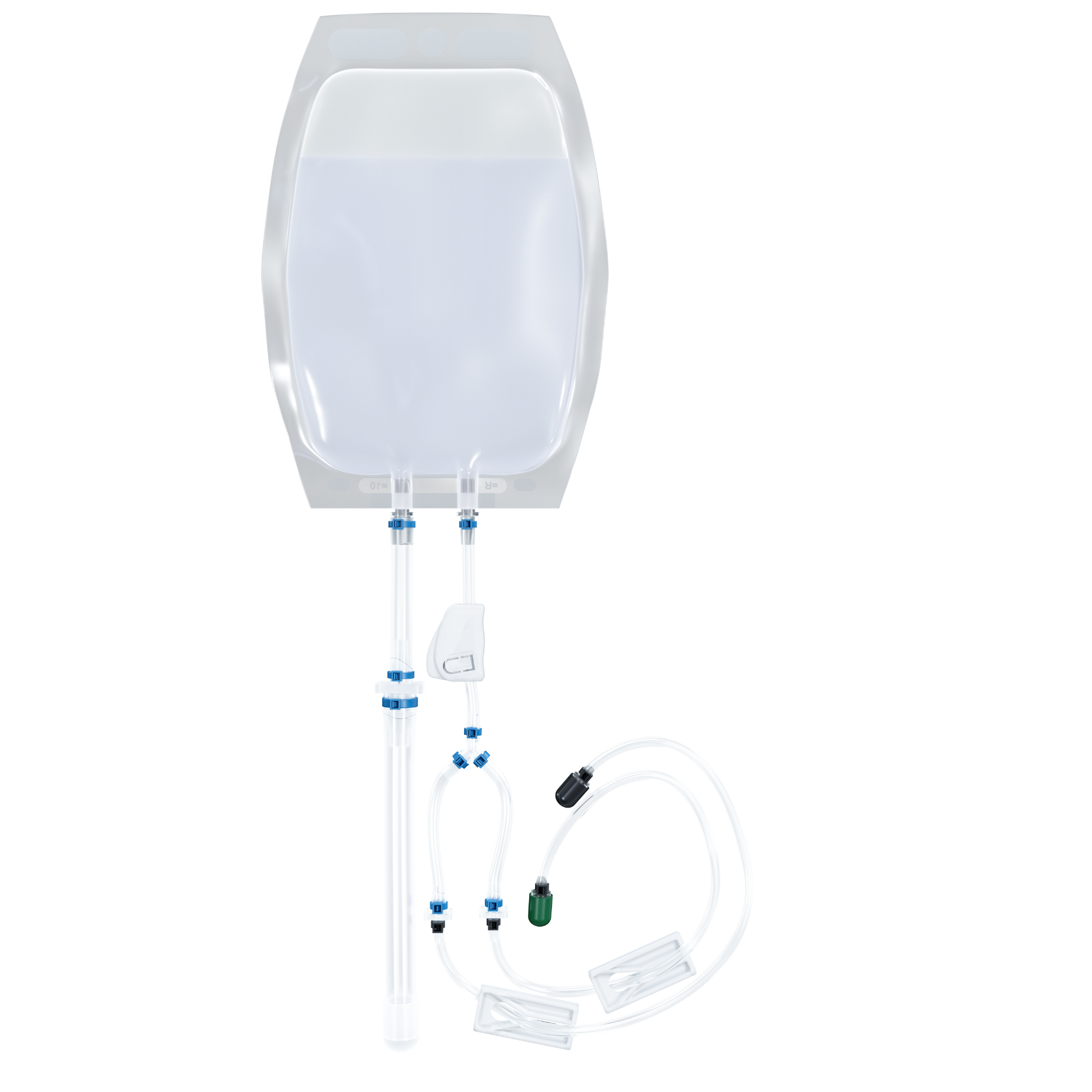
Image Credit: MaxCyte, Inc.
CL-2 GMP
This Flow Electroporation-enabled processing assembly allows users to clinically generate the therapeutic product up to a 100 mL working volume.
Features
- Closed systems and biowelding compatible
- Flow Electroporation permits the processing of up to 20 billion cells in 30 minutes
- Made of high-grade and inert material to safeguard previous cells

Image Credit: MaxCyte, Inc.
P×5 workflow rack
It supports, loads, manages, and transports up to five small processing units in a simple and secure way.
Features
- Durable, stackable, low footprint
- Withstands common lab disinfectants
- “To Do” and “Done” positions for tracking PA workflow status
- Contains 1 to 5 small volume PAs (≤1 ml)

Image Credit: MaxCyte, Inc.
Research applications
Cell therapy
Discover scalable engineering to allow safe and effective cell therapies.
Antibody and protein production
Transient expression for gram-scale protein synthesis can hasten the development of biotherapeutics.
Gene editing
Overcome the challenges associated with genome engineering with highly efficient delivery.