Accurate viral vector characterization in gene therapy is essential to achieving therapeutic results. The empty-to-full capsid ratio is one critical quality attribute (CQA) affecting gene therapy treatments' safety and effectiveness. The Vericheck ddPCR Empty-Full Capsid Kit from Bio-Rad precisely measures DNA and protein in a single well using the unmatched precision of Droplet DigitalTM PCR (ddPCR).
The entire adeno-associated virus (AAV) production workflow can be expedited by using the same assay for crude and purified samples and producing results daily. With this ground-breaking invention, the Vericheck ddPCR Empty-Full Capsid Kit streamlines the AAV development to produce dependable results. Using ddPCR technology, obtaining absolute quantification for different AAV serotypes is simple and quick.
- High-precision
- High precision of as low as 2% CV
- Easy data analysis
- Capsid and genome measurement via ddPCR technology
- Fast turnaround time
- Faster and higher throughput of 8 hour/14 samples
- Low sample input
- Minimal sample input of 2 μl
- Multi-sample compatibility
- For crude lysate and purified samples
Method overview
The Vericheck ddPCR Empty-Full Capsid Kit method combines two well-known methods—PCR and immunochemistry—to simplify testing. The kit allows for purified and crude lysate samples, increasing testing flexibility. It produces data from a single assay, such as the percentage of full capsids, vector genome titer, and capsid titer.
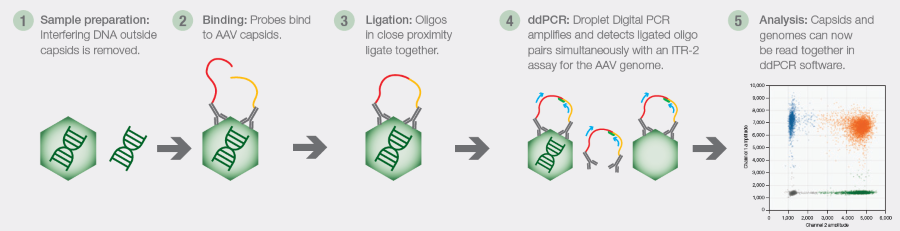
Fig 1. The Vericheck ddPCR Empty-Full Capsid Kit method. DNase treatment removes external DNA. Probes then specifically bind to AAV capsids. Oligos on these probes ligate together when in close proximity. ddPCR technology then amplifies and detects the ligated oligo pairs, while an ITR-2 assay simultaneously detects the AAV genome. Software analyzes the signals from each capsid type to calculate the empty-to-full capsid ratio. Image Credit: Bio-Rad Laboratories
- Exogenous DNA removal: Interfering DNA outside of capsids is eliminated using DNase
- Binding and ligation: Antibody probes with tethered oligos bound AAV capsids. Oligos that are near each other ligate.
- Droplet generation and amplification: ddPCR technology is used to create droplets and amp up and detect the ligated oligo pairs. Inverted terminal repeats (ITRs) are simultaneously detected by the ddPCR AAV ITR-2 assay to identify the AAV genome.
- Analysis and detection: The empty-to-full ratio is produced following the precise quantification of capsids and genomes
How ddPCR technology quantifies the empty-full capsid ratio
Fluorescence detection in FAM and HEX channels is used in the duplex assay Vericheck ddPCR Empty-Full Capsid Kit. FAM-labeled probes attach to capsid oligos during PCR, whereas HEX-labeled probes attach to the vector genome. Droplets consequently settle into one of four unique fluorescence clusters.
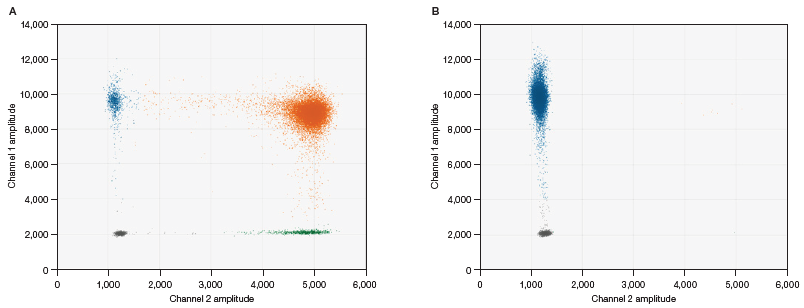
Fig 2. 2-D amplitude plot for comprehensive ddPCR analysis. The two-channel assay detects fluorescent signals from FAM and HEX dyes. Results are grouped into four main clusters: blue for empty AAV capsid signal (•), orange for full AAV capsid contents containing therapeutic genes (•), gray for negative droplets (•), and green for genome signals (•). A, full AAV capsid sample; B, empty AAV capsid sample. Image Credit: Bio-Rad Laboratories
High precision regardless of sample type with ddPCR technology
The Empty-Full Capsid Kit works with crude lysate and purified samples from any touch point during the production of AAV. The kit enables high precision of major AAV serotypes with only 2 µl of sample needed.
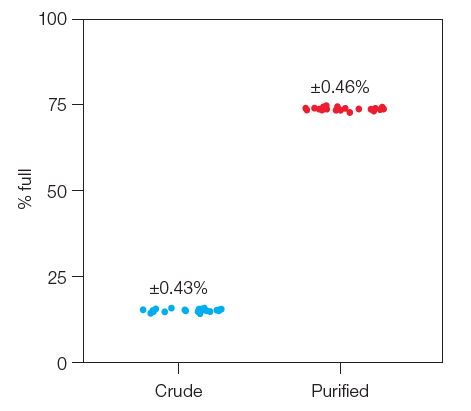
Fig 3. Low coefficient of variation (CV) for AAV serotype 9 (AAV9). The Vericheck ddPCR Empty-Full Capsid Kit was used to evaluate crude cell lysate and purified AAV. The results demonstrate consistent and precise measures of the percentage of full capsids. Note that no additional steps are required to measure crude samples. Crude samples (•); purified samples (•). Image Credit: Bio-Rad Laboratories
Highly reproducible with a shorter turnaround time
Within 8 hours, the Vericheck ddPCR Empty-Full Capsid Kit yields remarkably consistent results across various samples, users, lots, instruments, and days.
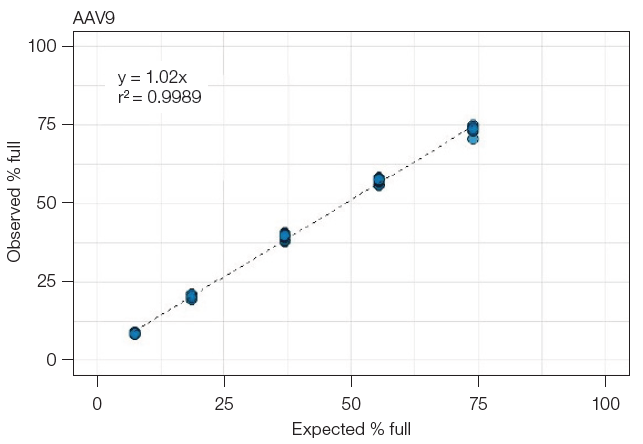
Fig 4. Repeatability across the measuring range. An AAV sample with predominantly filled capsids was mixed with empty capsids in varying ratios to create five samples across the measuring range. Each point represents a single test, with the dotted line indicating the linear regression analysis. The results show a consistent linear relationship between the expected and observed percentages of full capsids, from low to high levels, with high precision maintained throughout the range. Image Credit: Bio-Rad Laboratories
Higher accuracy with ddPCR technology
According to recent research, the contaminant DNA contained in AAVs may be counted as full capsids in analytical ultracentrifugation (AUC) but not in ddPCR techniques (Lecomte et al. 2015, McColl-Carboni et al. 2024, Schnödt et al. 2016). Droplet Digital PCR amplifies particular genomic regions to determine the amount of viral DNA in particles.
Specifically, ddPCR technology measures genome titer, capsid titer, and the percentage of full capsids in a single test, without external standard curves. On the other hand, AUC separates particles based on their sedimentation rates, providing detailed physical characterization (such as size, shape, and mass) without a reference standard.
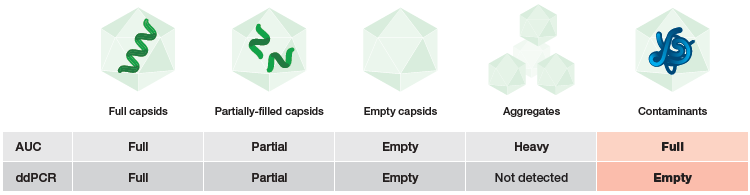
Fig 5. With ddPCR technology, only ITR-2 is detected, not contaminants, ensuring accurate categorization of full capsids. Analytical ultracentrifugation (AUC) differentiates empty and full capsids by mass, whereas the ddPCR method is specific to capsid content. Content-blind methods like AUC overestimate the number of therapeutic capsids by counting contaminants as full. As shown, capsids containing residual plasmid DNA are categorized full by AUC but negative by the ddPCR method. Additional residual DNA from host cell DNA may account for further differences. Image Credit: Bio-Rad Laboratories
Performance of ddPCR versus other technologies
The ddPCR method simplifies AAV development due to its high precision and ability to process crude lysate and purified samples. The empty-to-full ratio can be measured at any stage of AAV production using as little as 2 µl of sample.
Kits
Vericheck ddPCR Empty-Full Capsid Kit (AAV5). Image Credit: Bio-Rad Laboratories
Vericheck ddPCR Empty-Full Capsid Kit (AAV9). Image Credit: Bio-Rad Laboratories
Source: Bio-Rad Laboratories
| . |
. |
. |
| QX ONE Droplet Digital PCR (ddPCR) System |
 |
The QX ONE ddPCR System is designed to deliver a precise and multiplexed digital PCR system. This system seamlessly integrates a standard ddPCR workflow of droplet generation, thermal cycling, droplet reading, and analysis into a hands-free precision platform. |
| QX200 Droplet Digital PCR System |
 |
The QX200 Droplet Digital PCR (ddPCR) System provides absolute quantification of target DNA or RNA molecules for EvaGreen or probe-based digital PCR applications. |
| QX600 Droplet Digital PCR System |
 |
The QX600 Droplet Digital PCR System (ddPCR™) offers advanced six-color multiplexing and absolute quantification of nucleic acid, while delivering the best-in-class performance users have come to expect from Bio-Rad’s ddPCR systems. |
| QX600 AutoDG Droplet Digital PCR System |
 |
The QX600 AutoDG Droplet Digital PCR System (ddPCR) offers workflow automation, delivers advanced six-color multiplexing, and absolute quantification of nucleic acid without sacrificing the best-in-class performance you have come to expect from Bio-Rad’s ddPCR systems. |
| QX200 AutoDG Droplet Digital PCR System |
 |
The QX200 AutoDG Droplet Digital PCR System provides absolute quantification of target DNA or RNA molecules for EvaGreen and probe-based Droplet Digital PCR (ddPCR) applications. The Automated Droplet Generator simplifies the ddPCR workflow, making digital PCR both scalable and practical. |