Mass photometry is a groundbreaking technique for analyzing molecules. It allows us to measure the masses of individual molecules in solution precisely, in their natural state, and without labels.
A TwoMP mass photometer provides a wide mass range and single-molecule resolution for molecular mass measurements with unparalleled sensitivity, speed, and ease of use.
Benefits
- Easy setup – A compact, benchtop instrument with minimum installation requirements
- High-fidelity measurements of molecular mass
- Intuitive acquisition and data analysis software
- Minimal quantities of samples required

The TwoMP is ideal for the study of protein interactions, oligomerization, and macromolecular assembly. Image Credit: Refeyn

The TwoMP makes biomolecular characterization an easy task – requiring only tiny sample amounts. Image Credit: Refeyn
High-fidelity measurements
The TwoMP is perfect for measurements at physiological (low) concentrations because of its high sensitivity. The high dynamic range inherent in single molecule counting techniques ensures that low-abundance species are captured accurately.
Characterization of low-affinity interactions can be accomplished via MassFluidix HC, an add-on microfluidics system which significantly expands the range of sample concentrations that can be investigated by Mass Photometry. The MassFluidix HC system raises the upper concentration limit from nanomolar to micromolar range.
Intuitive and efficient
The Refeyn software automatically manages the acquisition procedure and completes the mass analysis in minutes. Users can interpret the mass distribution results intuitively without any prior knowledge.
Minimal setup required
It is a small, bench-top device that requires little installation. Only a few microliters of sample and clean sample-carrier slides are required for measurements.
Key applications of the TwoMP
Protein-protein interactions
Mass photometry can be used to study molecular systems and determine binding affinities for both mono- and multivalent interactions. One such example of a molecular system that can be studied using mass photometry is antigen-antibody interactions.
The figure below demonstrates the application of mass photometry in quantifying molecular interactions. By measuring the masses of the antibody trastuzumab and its target antigen, Her2, both individually and in mixtures, the interactions between individual antibody molecules and target antigens can be accurately quantified.
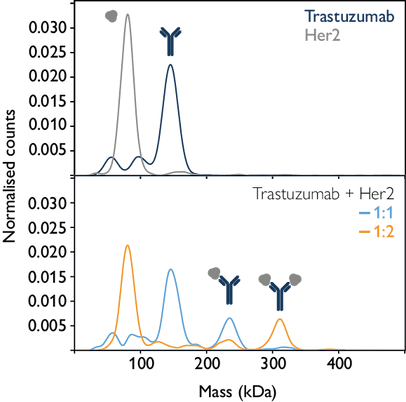
Image Credit: Refeyn
DNA-protein interactions
DNA-protein complexes, which are essential for gene expression, DNA replication, and DNA repair, can be characterized using mass photometry.
In the figure below, mass photometry not only enables the detection of DNA binding but also provides insights into how the protein's oligomeric state changes upon binding to DNA.
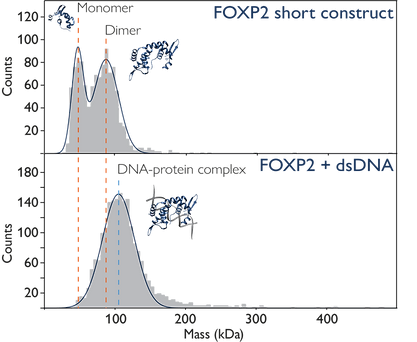
Image Credit: Refeyn
Protein oligomerization
Many proteins, in specific situations, take on a specific oligomeric form.
In the figure below, mass photometry characterizes four different proteins: protein A, beta-amylase, urease, and thyroglobulin. This characterization reveals a spectrum of behaviors, from purely monomeric (as seen with protein A) to dynamic equilibria between multiple states.
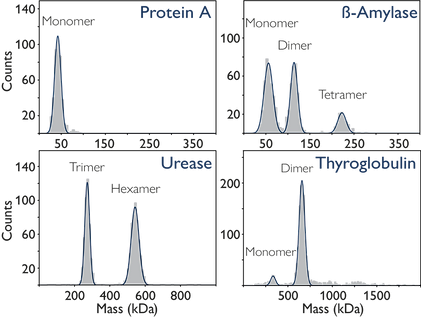
Image Credit: Refeyn
Complex formation
Central sites for protein synthesis are called ribosomes, which are macromolecular assemblies made of RNA and protein. The ribosomes of bacteria, in this case from E. coli, have a size greater than two MDa.
Magnesium ions play a subtle but crucial role in the assembly of intact (70S) complexes. In the absence of magnesium, E. coli ribosomes rapidly disassemble into their 30S and 50S subunits. Mass photometry allows us to monitor these processes (Figure).
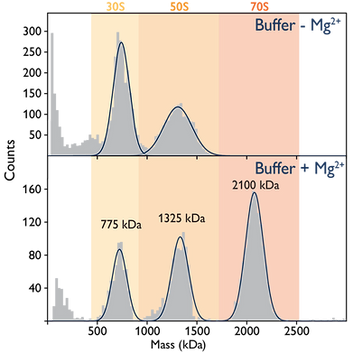
Image Credit: Refeyn