The MicroCal PEAQ-DSC Automated system provides high throughput, extremely sensitive protein stability analysis on an automated, integrated platform for greater productivity. For walk-away operations, all cell filling and cleaning procedures are entirely automated.
MicroCal PEAQ-DSC Automated data gives crucial direction for biopharmaceutical advancement in protein engineering, (pre)formulation development, process development, manufacturing change control, and biosimilarity and biocomparability studies.
The integrated software optimizes workflows and allows non-subjective data analysis, performance qualification, and adherence to 21 CFR Part 11 and Annex 11 regulations while delivering high-integrity data and boosting productivity in biopharmaceutical research. The unattended operation allows for 24-hour screening.

Image Credit: Malvern Panalytical
Live launch of Malvern MicroCal PEAQ-DSC systems
Video Credit: Malvern Panalytical
Features and benefits
One effective analytical method for determining the thermal stability of proteins and other biomolecules is differential scanning calorimetry (DSC). This method measures the temperature (Tm) and enthalpy (ΔH) of thermally induced structural changes within molecules in solution.
This information offers a critical understanding of the dynamics that stabilize or destabilize nucleic acids, proteins, micellar complexes, and other macromolecular systems.
The information is utilized in the development of purification strategies, the characterization and assessment of protein constructs, the ranking of ligand affinities to their protein targets in small molecule drug discovery programs, and the prediction of shelf-life of biomolecular products, including biopharmaceuticals, to facilitate batch-to-batch and biosimilar versus innovator molecule comparisons.
The software on the high-throughput, high-sensitivity MicroCal PEAQ-DSC Automated system simplifies processes with variable instrument scheduling and simpler experimental setup. Automated data analysis makes it simple to integrate into current data handling and transmission systems while supporting producing high-quality thermal stability data and ensuring compliance with legal standards.
Key features
- The gold standard for stability-indicating methods that need minimal assay development
- Measurement of biomolecular native-state stability in solution in a direct and label-free manner
- Conventional 96-well plate format for large capacity and simple loading; up to 6 plates can be stored with temperature control
- Up to 50 samples can be analyzed unattended each day with a fully integrated autosampler
- Automated features for cleaning and filling cells enable unsupervised operation
- Extremely tight binding constant measurement, up to 1020M–1
- Strong MicroCal PEAQ-DSC software contains the following features and reduces usual data analysis time:
- PEAQ-Compliance: Malvern Access Controller (MAC) is a feature of the system that allows access to user-defined SOPs and data analysis functions to be restricted. To help comply with 21 CFR Part 11 and Annex 11 standards and to make integrating the workflow into the company’s quality system easier, data can be shown using the Report Generator along with user details and the option to sign using an electronic signature. This is offered as an add-on item
- PEAQ-Performance: Identifies and verifies system readiness automatically for optimal performance
- PEAQ-Smart (including PEAQ-Finder): For data analysis and SOP-based operations. Offers novel algorithms capable of identifying even the most minute peaks and shoulders, making it easier to automatically and objectively identify many transitions—like those seen in multi-domain proteins
- PEAQ-Compare: Perfect for batch-to-batch and biosimilarity investigations, for the quantitative analysis of DSC traces for comparability studies
- Network-ready: Email updates are supplied during the analysis to keep users up to speed on the status of the test
The MicroCal PEAQ-DSC is also available without an autosampler as a manual system.
How it works
Temperature-induced conformational changes, such as unfolding, are common in functional structures generated by proteins and other macromolecules. As a result of the rearrangement of non-covalent bonds within the molecule, these changes result in heat absorption. Differential Scanning Calorimeters precisely detect this heat uptake.
The MicroCal PEAQ-DSC Automated system’s thermal core has a sample cell (containing the sample of interest) and a reference cell (containing a matched buffer solution), both enclosed within an insulating jacket. These two cells are constantly kept at the same temperature and heated at a constant scan rate during measurements.
Heat is absorbed when the molecule within the sample cell unfolds, resulting in a temperature differential (ΔT) between the sample and the reference cells. This creates a thermal gradient across the Peltier units, producing a proportional voltage. This is then translated to power to form a feedback loop to the Peltier units and restore ΔT to zero.
Since protein unfolding is an endothermic process, it appears as a positive displacement on the thermogram. The Tm represents the midpoint of this protein ‘melting’ transition, and the area under the curve indicates the unfolding process’s enthalpy (ΔH).
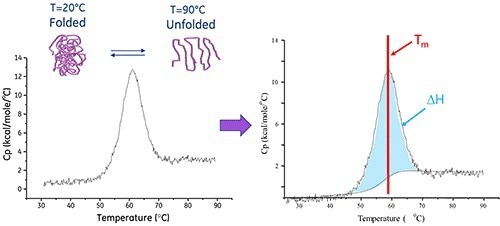
Image Credit: Malvern Panalytical
Specification
Source: Malvern Panalytical
| General |
|
| Technology |
Differential Scanning Calorimetry |
| Measurement type |
Temperature midpoint (TM)
Enthalpy (ΔH)
Heat capacity change (ΔCp) |
| Cell |
|
| Cell |
Capillary |
| Cell material |
Tantalum |
| Cell volume |
130 μL |
| Sample |
|
| Sample capacity |
288 (6 × 96-well plates) |
| Sample volume |
325 µL |
| Typical sample concentration |
0.01 mg/mL – 10 mg/mL 1 |
| Sample throughput |
≤50 samples/day |
| Sample storage temperature range |
4 °C - 40 °C |
| System |
|
| Noise |
0.05 μCal/°C 2 |
| Baseline repeatability |
1 μCal/°C 2 |
| Response time |
5s 2 |
| Repeatability |
<0.2 µCal/°C 3 |
| Reproducibility |
<0.08 °C St. Dev. TM and <2% RSD on ΔH 4 |
| System reproducibility |
<0.1 °C St. Dev. TM and <5% RSD on ΔH 4 |
| Multiple feedback modes |
Yes (passive, high gain and low gain) |
| Temperature range |
2 °C to 130 °C 2, 5 |
| Maximum scan rate |
240 °C/h |
| Reverse scanning |
Yes |
| Pressure perturbation calorimetry (PPC) |
N/A |
| Cleaning routines |
3 pre-programmed routines |
| Cleaning solvents |
Water and detergent used as standard |
| Software |
|
| 21 CFR part 11 |
Yes, with PEAQ-Compliance software option |
| Network ready |
Yes, with email alert capability |
| Operating environment |
|
| Operating temperature (°C) |
10 °C to 28 °C |
| Storage temperature |
-20 °C to 50 °C |
| Humidity |
10% to 70%, non-condensing (10% to 90% for storage) |
| Ingress Protection (IP) rating |
IP21 |
| Power |
100-240 V A/C, 50/60 Hz, 70 W (cell), 400 W (max, autosampler), PC as supplied |
| Certification |
CE (EN61010-1), EMC (EN61326-2-1, EN61326-1, FCC, ICES, VCCI), ISO9001:2008 |
| Weight and dimensions |
|
| Dimensions (W, D, H) |
101 cm × 68 cm × 70 cm |
| Weight |
Approx. 25 kg |
Notes
1 Sample dependent
2 Typical results for ribonuclease (RNase) in 50 mM KAc buffer at pH 5.5, at 60 °C/h with high feedback
3 Rescans of a stable buffer
4 Using ribonuclease (RNase)
5 Range may be extended down to -10 °C upon request
Key applications
Biotherapeutic discovery, development and manufacturing
The MicroCal PEAQ-DSC Automated is widely used in the biopharmaceutical sector to give vital stability data for biotherapeutics in the research, development, and production stages. This is due to the detection method’s universality, the capacity to detect extremely tiny changes in the structure of biological drugs, and the data’s excellent repeatability.
Biosimilarity and biocomparabilty
The extremely reproducible characteristics of the MicroCal PEAQ-DSC devices and the technique's capacity to identify alterations to a single amino acid in a protein have been the primary reasons behind the use of DSC data in biosimilar medicine submissions. A biosimilarity function is included in the MicroCal PEAQ-DSC program for statistical comparison with the innovator drug.
Characterization of antibody drug conjugates and vaccines
DSC, DLS, and other biophysical techniques can be used to investigate the stability of Antibody Drug Candidates, in addition to the impact of antibody drug ratios on their stability and proclivity to aggregate. The next example demonstrates how DSC can determine the inactivation kinetics by heating over various scan rates.
In the current state DSC is one of the key approaches to the successful development of biopharmaceuticals.”
Sorina Morar-Mitrica, PhD, GlaxoSmithKline
The “Gold Standard” for structural stability analysis of biotherapeutics, biological macromolecules and polymers in solution
Image Credit: Malvern Panalytical
The MicroCal PEAQ-DSC Automated provides tools for determining the thermal stability of proteins and other biomolecules in biopharmaceutical research and manufacturing. It is easy to use, requires little assay development, and does not need labeling or immobilization.