To test for the presence of bilirubin in urine, Ictotest Reagent Tablets are utilized. The existence of bilirubin is a significant finding in the assessment of liver function, and a positive detection can be a sign of liver disease, such as cirrhosis, jaundice, or hepatitis. Laboratories and hospitals depend on the quality of Siemens’ Ictotest reagent tablets to verify bilirubin outcomes before offering them to customers.
- Ictotest Reagent Tablets were initially made available in 1953
- Use of Ictotest needs a dropper and distilled water, besides the absorbent mats and reagent tablets offered.
- Level of detection ≥0.05 to 0.1 mg bilirubin/dL in urine (0.9–1.7 µmol/L).
- The simple test provides timely and precise confirmatory urine bilirubin outcomes in just one minute.
Icotest Reagent Tablets. Image Credit: Siemens Medical Solutions USA, Inc
Features and benefits
Why is a confirmatory test essential for urinary bilirubin? So far, no quantitative urine bilirubin test method is commercially available. The only way to exclude urine-strip false-positive bilirubin outcomes is with a confirmatory assay like Ictotest tablets. The substitute is for the healthcare professional to order a blood test for bilirubin, which increases the time and cost of the confirmation process.
Usage of Ictotest tablets needs a pipette and distilled water, besides the absorbent mats and reagent tablets offered with the Ictotest kit. It is simple to perform the procedure and provides trustworthy answers when a fresh urine specimen is utilized and the tablets are stored correctly.
Clinical use
The Ictotest tablets, depending on the diazotization reaction, are used as a confirmatory assay for the existence of urinary bilirubin when a frontline test (urine strip or another semi-quantitative technique) has offered a positive bilirubin outcome. The Ictotest reagent tablets could be utilized to rule out the existence of interfering substances that might result in a false positive outcome.
- Users can place a square of the absorbent test mat that has been supplied onto a paper towel. Utilizing either side of the test mat will give the anticipated outcomes. Place 10 drops of urine onto the center of the test mat.
- Shake one Ictotest Reagent Tablet into the bottle cap and shift the tablet to the center of the moistened mat. Users should not handle the tablet with their fingers. Recap the bottle quickly.
- Place a single drop of distilled water onto the tablet. Then, wait five seconds and place a second drop of distilled water onto the tablet so that the distilled water departs the tablet onto the mat.
- Note the color of the mat around and under the tablet at 1 minute.
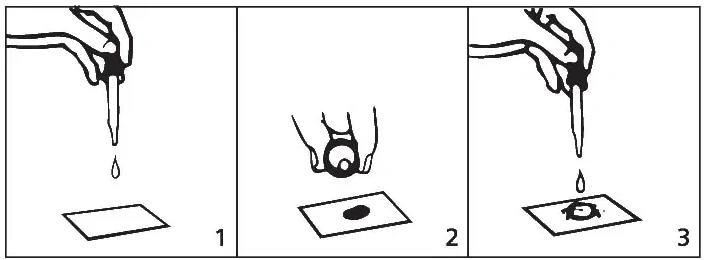
Image Credit: Siemens Medical Solutions USA, Inc
Results: Results with Ictotest Reagent Tablets come as negative if no purple or blue color develops on the mat in just a minute. If a blue or purple color form on the mat or under the tablet within 60 seconds, the result is taken as positive. Pink or red color must be overlooked.
Positive Result: A purple or blue color on the mat denotes the presence of bilirubin. The illustration at left displays normal positive results. The color gradient shows a sample range of colors for a positive result.
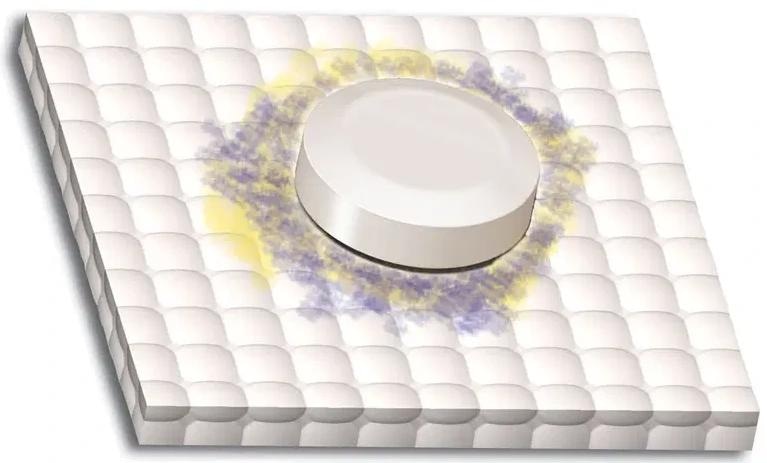
Image Credit: Siemens Medical Solutions USA, Inc
Negative results: The absence of purple or blue on the mat indicates no bilirubin. A light red or pink color must be ignored. The demonstration at left displays the expected negative results. The color gradient shows a sample range of colors for a negative result.
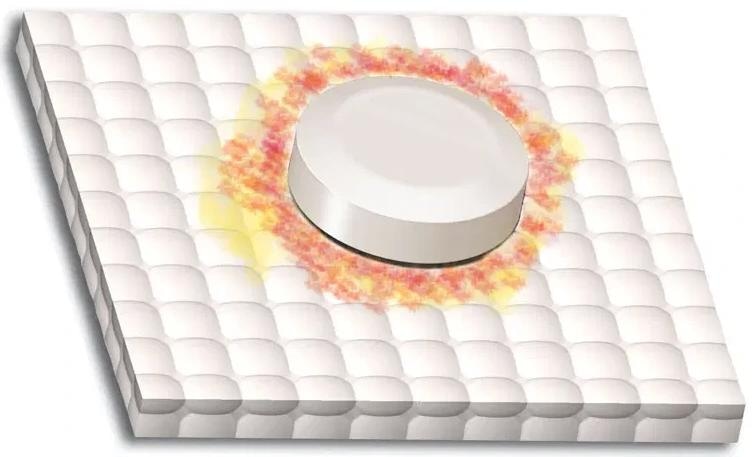
Image Credit: Siemens Medical Solutions USA, Inc
Technical specifications
Chemical principles of the procedure: The reaction relies on coupling a special solid diazonium salt with bilirubin in an acid medium to give the purple or blue reaction.
Storage and handling: Ictotest Reagent Tablets are steady until the expiration date in the unopened container if stored at temperatures between 15 and 30 °C. Users should not store the bottle in direct sunlight. Replace the cap quickly and tightly after use. Do not use tablets that are broken.
Limitations of the procedure: Metabolites of (phenazopyridine) provide bright red-orange colors, which might mask the reaction of small amounts of bilirubin. High concentrations of urobilinogen do not conceal the reaction of small amounts of bilirubin, but atypical orange colors are created.
Chlorpromazine in huge amounts might provide a false positive outcome, and metabolites of Lodine (etodolac) may result in false positive or atypical results.
Specific Performance Characteristics: Ictotest Reagent Tablets will have the potential to detect as little as 0.05 to 0.1 mg bilirubin/dL in urine (0.9 to 1.7 µmol/L).