Ischemic stroke is a condition in which parts of the brain lose their blood supply, causing nerve damage. Once brain tissue suffers irreparable harm, the patient will experience irreversible disability or death, depending on the extent of neuronal loss. However, glial cells surrounding the neurons are activated by the injury and multiply. Now, scientists have found out how to insert genes into glial cells to convert them into neurons, thus filling in for some of the lost functioning cells to improve motor functions.
There are about 86 billion neurons in the brain, but billions of them can be lost with one moderate-sized stroke. About 800,000 new strokes occur each year in the US alone. The need is to regenerate new brain cells to replace the ones that die, at least partially. This is the only known way to restore motor functions that have been impaired or destroyed by a stroke or other brain injury.
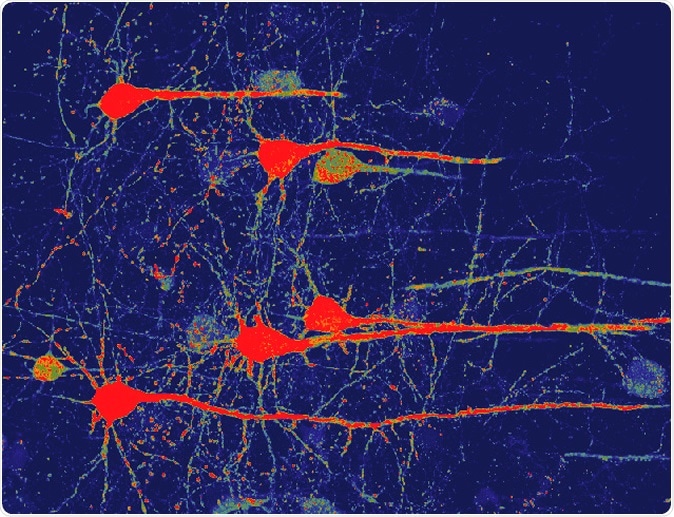
Image of neurons (red) that were converted from glial cells using a new NeuroD1-based gene therapy in a stroke-injured mouse brain. Credit: Chen Laboratory, Penn State
Older approaches relied on neural stem cells (NSCs) and transplanted neural progenitor cells (NPCs) from outside. While the latter showed promise, it has not produced a clinical therapy. The use of NSCs led only to reactive astroglial production rather than neurons. Moreover, NSCs from outside the body lead to various immune phenomena because of their immune identity. Various chemicals that induce neural growth have also been used.
Researcher Gong Chen says, “The biggest obstacle for brain repair is that neurons cannot regenerate themselves. Many clinical trials for stroke have failed over the past several decades, largely because none of them can regenerate enough new neurons to replenish the lost neurons.”
In this new study, the researchers used a new method: they took glial cells, the most abundant cell type in the brain. These glial cells have supportive and immune functions. They are found around all neurons in all brain locations. They remain capable of cell division and regeneration, and in fact, actively do so to heal the brain after an injury. This is how glial scars are formed in the brain after trauma.
Here, Chen and his team worked on glial cells already in the brain, the ones that neighbour the dead neurons, to turn them into nerve cells instead. The rationale is that the glial neighbors of a neuron probably stem from the same cell type, making the conversion easier.
Earlier research by the same team had shown how a gene encoding a neural transcription factor called NeuroD1 acted to turn mouse glia into mouse neurons, with functional nervous activity, inside the brains of mouse models with Alzheimer’s disease. It has been aptly called “a gene therapy-based cell therapy.” However, only a few cells were successfully converted. The reason for this was the ineptness of the retroviral vector used to deliver the therapeutic gene into the brain.
This time around, they opted for the AAV viral vector, which has been used in a host of similar experiments with great effectiveness. It infects both dividing and non-dividing cells with high efficacy. The gene was loaded into the AAV and thus inserted into the motor cortex of the brains of mice which had suffered a stroke, at 10 days post-stroke, when reactive astrocytosis was shown to occur.
This system was built to enable the expression of higher or significant levels of NeuroD1 in the injured areas, specifically inside the scar-forming glial cells, thus turning them into nerve cells instead. This means that the number of functioning neurons in the injured area shot up, and alleviated the loss of brain tissue due to the stroke. Overall, the team observed that one-third of the dead neurons were replaced by converted astroglia, while another one-third were protected against injury. This helped foster nervous recovery to a very large extent. The conversion was confirmed by RNA-sequencing techniques and immunostaining procedures. Motor tests like food pellet retrieval and grid walking were performed both before and after the ischemic injury, and showed a significant recovery of nerve function to 80% or more of the pre-injury level.
The new neurons were surprisingly similar to the old ones, which seems to show that the type of cell which gives rise to the glia affects the type of neurons formed by the converted glia.
Even better, the newly formed neurons actually function as they are supposed to, generating action potentials (nerve impulses) and reaching out to form connections with other “original” neurons in synaptic networks. They grow long axons in the right direction to reach and make contact with the appropriate target cells, and altogether, the process helps to accelerate the recovery of motor functions in the injured mouse.
Meanwhile, a collaborative experiment was putting the NeuroD1 gene therapy through its paces in a rat model after suffering stroke. Here too, the conversion of glia to neurons resulted in an improvement in cognitive or thought-related issues that were caused by the stroke.
Another advantage is that even 10 days after the ischemic injury, efficient astrocyte conversion can occur, broadening the window of opportunity for treatment for patients in remote locations, or under-developed regions, for instance. Glial cells are ubiquitous in the brain, allowing an abundant source of cells which can be used to regenerate neurons.
Another researcher, Yuchen Chen, says, “Because glial cells are everywhere in the brain and can divide to regenerate themselves, our study provides the proof-of-concept that glial cells in the brain can be tapped to regenerate functional new neurons for brain repair not only for stroke but also for many other neurological disorders that result in neuronal loss. Our next step is to further test this technology and ultimately to translate it into clinically effective therapies to benefit millions of patients worldwide.”
The paper appeared online in the journal Molecular Therapy.
Journal reference:
A NeuroD1 AAV-based gene therapy for functional brain repair after ischemic injury through in vivo astrocyte-to-neuron conversion. Yu-Chen Chen, Ning-Xin Ma, Zi-Fei Pei, Zheng Wu, Fabricio H. Do-Monte, SusanKeefe, Emma Yellin, Miranda S. Chen, Jiu-Chao Yin, Grace Lee, Angélica Minier-Toribio, Yi Hu, Yu-Ting Bai, Kathryn Lee, Gregory J. Quirk, & Gong Chen. Molecular Therapy (2019). https://doi.org/10.1016/j.ymthe.2019.09.003