2. What should I know before I use TIBSOVO?
|
WARNING: DIFFERENTIATION SYNDROME AND QTc INTERVAL PROLONGATION.
TIBSOVO can cause Differentiation Syndrome in patients with AML, which is a severe
reaction to medicines used to treat leukaemia . Symptoms may include: fever; cough;
trouble breathing; weight gain; rash; decreased urination; dizziness or light-headedness;
rapid weight gain; and swelling of the arms, legs, and neck. TIBSOVO can also cause
QTc Interval Prolongation, regardless of the type of cancer a person has, which can
cause life-threatening irregular heartbeats. Symptoms may include feeling dizzy, light-headed
or faint. These conditions can be life-threatening or lead to death if not treated.
Seek urgent medical attention or go straight to the Emergency Department at your nearest
hospital if you notice any of these symptoms.
|
Do not use if you have ever had an allergic reaction to TIBSOVO or any of the ingredients
listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section
2. What should I know before I use TIBSOVO? in the full CMI.
3. What if I am taking other medicines?
4. How do I use TIBSOVO?
The usual dose of TIBSOVO is two tablets to be taken once daily at approximately the
same time each day. In some cases your doctor may tell you to take a reduced dose
if you are taking some other medicines or to help you better tolerate some possible
side effects. It is important to follow the instructions provided and use TIBSOVO
until your doctor tells you to stop. More instructions can be found in Section
4. How do I use TIBSOVO? in the full CMI.
5. What should I know while using TIBSOVO?
|
Things you should do
|
Remind any doctor, dentist, or pharmacist you visit that you are using TIBSOVO.
Continue regular monitoring (e.g. echocardiogram and/or blood tests) as directed by
your doctor.
|
|
Things you should not do
|
Do not take TIBSOVO along with a high-fat meal.
Do not have grapefruit or grapefruit juice during treatment with TIBSOVO as it can
affect how this medicine works.
You should not take TIBSOVO together with certain medications including dabigatran,
St.John’s wort, rifampicin or certain medicines used to treat epilepsy (e.g. carbamazepine,
phenobarbital, phenytoin).
|
|
Looking after your medicine
|
Store in a cool dry place away from moisture, heat, and sunlight. Keep out of reach
of children.
Close the bottle lid tightly to prevent moisture.
|
6. Are there any side effects?
TIBSOVO is usually well tolerated, however all medications may have unwanted effects
in some people. Always tell your healthcare provider about any side effects that you
experience. For more information, including what to do if you have any side effects,
see Section
6. Are there any side effects? in the full CMI.
This medicine is subject to additional monitoring. This will allow quick identification
of new safety information.
You can help by reporting any side effects you may get. You can report side effects
to your doctor, or directly at www.tga.gov.au/reporting-problems
Active ingredient(s):
ivosidenib
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using TIBSOVO. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using TIBSOVO.
Where to find information in this leaflet:
1. Why am I using TIBSOVO?
TIBSOVO contains the active substance ivosidenib.
TIBSOVO is used to treat specific cancers (bile duct cancer and acute myeloid leukaemia)
that contain a particular mutated (abnormal) form of the IDH1 enzyme.
When the IDH1 enzyme is mutated, metabolic changes in the cell can lead to the development
of cancer. TIBSOVO blocks the effects of the mutated enzyme and helps to slow or stop
the cancer from growing.
TIBSOVO can be used to treat adults with bile duct cancer (also known as ‘cholangiocarcinoma’).
It is used to treat patients whose bile duct cancer has spread to other parts of the
body and when therapy with other anti-cancer medicines are no longer working.
TIBSOVO can also be used alone or in combination with another anti-cancer medicine
called azacitidine, to treat adults with acute myeloid leukaemia (AML) who are not
eligible to receive intensive chemotherapy.
TIBSOVO can also be used alone in adults with AML when the disease has come back or
has not improved after previous treatment(s).
TIBSOVO is only used in patients whose cancer is related to a particular mutation
(called R132) in the IDH1 enzyme.
Your doctor will have performed a test confirming the cancer contains the R132-mutated
IDH1 enzyme before deciding that this medicine is the right treatment for you. Speak
to your doctor if you have any questions about this test and the results.
2. What should I know before I use TIBSOVO?
There are some people who shouldn't take TIBSOVO. Please read the list below. If you
think any of these situations apply to you or you have any questions, please see your
doctor.
Warnings
Do not use TIBSOVO if:
you are allergic to ivosidenib or any of the other ingredients of this medicine (listed
in section 3); or
you are already taking medicines such as dabigatran, St.John’s wort, rifampicin or
certain medicines used to treat epilepsy (e.g. carbamazepine, phenobarbital, phenytoin).
Precautions when taking TIBSOVO:
If you have AML, and if your cancer responds to treatment, TIBSOVO can cause a serious
condition known as Differentiation Syndrome, which can be life-threatening if not
treated. Seek urgent medical attention if you develop fever; cough; trouble breathing;
weight gain; rash; swelling of the arms, legs, and neck; build-up of excess fluid
around the heart and lungs; or low blood pressure (see "Serious side effects" in Section
6. Are there any side effects?).
TIBSOVO can cause a serious condition known as QTc interval prolongation, which can
cause life-threatening irregular heartbeats. Seek urgent medical attention if you
feel dizzy, light-headed or faint (see also Section 6. Are there any side effects?)
after taking TIBSOVO.
Guillain-Barré syndrome has happened in patients treated with TIBSOVO. Your doctor
will monitor you for nervous system problems. Tell your doctor if you develop weakness
or tingling feeling in your legs, arms, or upper body, numbness and pain on one side
or both sides of your body, any changes in your ability to see, touch, hear, or taste,
burning or prickling sensation, or difficulty breathing.
If you get any of the above serious side effects, your doctor may give you other medicines
to treat them and they may tell you to stop taking TIBSOVO for a while or stop taking
it altogether.
Check with your doctor if:
you have heart problems including a condition called long QT syndrome or have problems
with abnormal electrolyte levels (such as sodium, potassium, calcium or magnesium);
you are taking certain medicines that can affect the heart (e.g. those used to prevent
arrhythmia called anti-arrhythmics, some antibiotics, some antifungals and those used
to prevent nausea and vomiting - see also Section
3. What if I am taking other medicines?);
you have kidney problems; and/or
you have liver problems.
Other side effects that could happen during treatment are described under Section
? It is important you understand these risks and how to look out for them.
Pregnancy and breastfeeding
It is important to tell your doctor if you are pregnant or intend to become pregnant,
or if you are breastfeeding or intend to breastfeed.
Pregnancy
TIBSOVO is not recommended for use during pregnancy as it may harm the unborn baby.
People of child-bearing age should have a pregnancy test prior to starting treatment
with TIBSOVO and should avoid becoming pregnant during therapy.
If you are pregnant, think you might be pregnant or are planning to have a baby, ask
your doctor for advice before taking this medicine. Contact your doctor or nurse immediately
if you become pregnant whilst taking TIBSOVO.
As TIBSOVO may affect an unborn developing baby, it is important for women not to
become pregnant for at least 1 month after treatment discontinuation.
Contraception
People who might become pregnant, and people who have partners who might become pregnant,
should use effective contraception during treatment with TIBSOVO and for at least
1 month after the last dose.
TIBSOVO may stop hormonal contraceptives from working properly. If you or your partner
use a hormonal contraceptive (e.g. birth control pills, or contraceptive patches or
implants), you should also use a barrier method (e.g. condoms or a diaphragm) to avoid
pregnancy. Talk to your doctor or nurse about the right contraceptive method for you.
Breastfeeding
It is not known if TIBSOVO passes into breast milk. Do not breastfeed your baby during
treatment with TIBSOVO and for at least 1 month after the last dose.
Fertility
It is not known if TIBSOVO affects fertility. If you are concerned about your fertility
whilst taking TIBSOVO talk to your doctor.
Children and adolescents
Do not give this medicine to children and adolescents under 18 years old because there is
no information about its use in this age group.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
This is because they may reduce how well TIBSOVO works or increase the risk of side
effects, or TIBSOVO may affect the way these other medicines work.
In particular, tell your doctor if you are taking any of the following medications:
antibiotics used for bacterial infections (e.g. erythromycin, clarithromycin, benzylpenicillin,
ciprofloxacin, levofloxacin);
warfarin (used to stop blood clots);
medicines used for fungal infections (e.g. itraconazole, ketoconazole, fluconazole, isavuconazole, posaconazole, voriconazole);
medicines that affect your heartbeat known as anti-arrhythmics (e.g diltiazem, verapamil, quinidine);
medicines used to stop nausea and vomiting known as anti-emetics (e.g aprepitant, ondansetron, tropisetron, granisetron);
medicines used after organ transplants known as immunosuppressants (e.g. ciclosporin, everolimus, sirolimus, tacrolimus);
medicines used for HIV (e.g. raltegravir, ritonavir);
alfentanil (used for anaesthesia in surgery);
fentanyl (used for severe pain);
pimozide (used for schizophrenia);
medicines used for cancer (e.g. cyclophosphamide, ifosfamide, paclitaxel);
methadone (used for morphine or heroin addiction, or severe pain);
medicines used for type 2 diabetes (e.g. pioglitazone, repaglinide);
omeprazole (used for stomach ulcers and acid reflux);
furosemide (used for fluid build-up known as oedema);
medicines used for high cholesterol known as statins (e.g. atorvastatin, pravastatin, rosuvastatin); and/or
lamotrigine (used for epilepsy).
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect TIBSOVO.
4. How do I use TIBSOVO?
How much to take / use
Always take this medicine exactly as your doctor has instructed. Check with your doctor
if you are unsure.
The recommended dose is 2 tablets (500 mg ivosidenib) to be taken once daily at approximately the same time each day.
Your doctor may tell you to take 1 tablet (250 mg ivosidenib) if you are taking some other medicines or to help you better tolerate some possible side effects. Follow the dosage instructions from your doctor.
The tablets can be taken with or without food, but do not take it with fatty foods
(e.g. high fat breakfast).
Swallow the tablets whole with water.
If you vomit after taking your usual dose, do not take additional tablets. Take your
next dose as usual the following day.
It is important to follow the instructions provided and use TIBSOVO until your doctor
tells you to stop.
When to take / use TIBSOVO
TIBSOVO should be taken once daily at approximately the same time each day.
If you forget to take TIBSOVO
If a dose is missed or not taken at the usual time, take the tablets as soon as possible
unless the next dose is due within 12 hours.
Do not take two doses within 12 hours.
Take the next dose as usual the following day.
If you use too much TIBSOVO
If you think that you have used too much TIBSOVO you may need urgent medical attention.
You should immediately
do one of the following:
phone the Poisons Information Centre
(by calling
13 11 26);
contact your doctor; or
go to the Emergency Department at your nearest hospital.
You should do one of the above even if there are no signs of discomfort or poisoning.
TIBSOVO with food and drink
Do not have grapefruit or grapefruit juice during treatment with TIBSOVO as it can affect
how this medicine works.
Do not take the tablets with fatty foods (e.g., high fat breakfast), as it can affect how this
medicine works
5. What should I know while using TIBSOVO?
Things you should do
Regular Tests
You will be monitored closely by your doctor before and during treatment with TIBSOVO.
You will need to have regular electrocardiograms (ECGs) to monitor your heartbeat,
in a hospital. An ECG is a recording of the electrical activity in your heart. You
will be given an ECG before you start treatment with TIBSOVO, once a week for the
first three weeks of treatment, and then once monthly thereafter.
Call your doctor straight away or go straight to the Emergency Department if you experience
any serious side effects.
Things you should not do
Do not stop using this medicine without consulting your doctor.
Driving or using machines
This medicine is not likely to affect your ability to drive or use any tools or machines,
but if you feel unwell after taking TIBSOVO, do not drive or use any tools or machines
until you feel well again.
Drinking alcohol
Tell your doctor if you drink alcohol.
Drinking alcohol could worsen certain side effects that you may have with TIBSOVO.
Looking after your medicine
Store it in a cool dry place away from moisture, heat or sunlight (e.g. high on a
pantry shelf away from the stove).
Do not store it in the refrigerator, bathroom, near a sink, in the car, or on windowsills.
Keep the bottle tightly closed in order to protect from moisture.
Follow the instructions in the carton on how to take care of your medicine properly.
Keep it where young children cannot reach it.
When to discard your medicine
Do not use this medicine after the expiry date which is printed on the bottle label
and box after EXP.
The expiry date is the last day of the printed month.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, please take it to
any pharmacy for safe disposal.
6. Are there any side effects?
All medicines can have side effects. Some side effects may need medical attention.
Ask your doctor or pharmacist if you have any further questions about side effects
after reading the below information.
Less serious side effects
Serious side effects
Tell your doctor or nurse if you notice anything else that may be making you feel
unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What TIBSOVO contains
|
Active ingredient
(main ingredient)
|
Ivosidenib
|
|
Other ingredients
(inactive ingredients)
|
Microcrystalline cellulose
Croscarmellose sodium
Hypromellose acetate succinate
Colloidal anhydrous silica
Magnesium stearate
Sodium lauryl sulfate
Hypromellose
Titanium dioxide
Lactose monohydrate
Triacetin
Indigo carmine aluminium lake
|
Do not take this medicine if you are allergic to any of these ingredients.
What TIBSOVO looks like
TIBSOVO tablets are blue, oval shaped, film-coated tablets approximately 18 mm in
length, debossed with 'IVO' on one side and '250' on the other side.
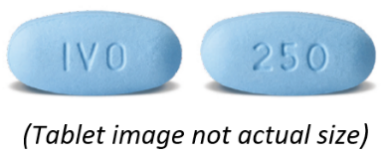
TIBSOVO is available in plastic bottles containing 60 tablets and a silica gel desiccant.
The bottles are packaged in a cardboard box; each box contains 1 bottle.
(AUST R 391874).
Who distributes TIBSOVO
Servier Laboratories (Aust.) Pty. Ltd.
Level 4, Building 9
588A Swan Street,
Burnley, 3121, Victoria, Australia
This leaflet was prepared in May 2024