2. What should I know before I use XOLAIR?
Do not use if you have ever had an allergic reaction to omalizumab or any of the ingredients
listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section
2. What should I know before I use XOLAIR? in the full CMI.
3. What if I am taking other medicines?
4. How do I use XOLAIR?
Your healthcare provider will prescribe the dose of XOLAIR that is right for you.
Use XOLAIR exactly as prescribed.
Do not try to inject XOLAIR yourself until you or your caregiver has been shown how
by your healthcare provider.
If you have forgotten to inject a dose of XOLAIR, do not take a double dose to make
up for the dose you missed.
5. What should I know while using XOLAIR?
|
Things you should do
|
Discontinue treatment and tell your doctor or pharmacist immediately if you get any
signs or symptoms of a potentially serious infection or an allergic reaction during
treatment with XOLAIR.
Tell your doctor you are taking XOLAIR before a vaccination as live vaccines are not
suitable for you.
|
|
Things you should not do
|
Do not stop taking your medicine or change dosage without checking with your doctor
first.
Do not use this medicine if the liquid contains easily visible particles, is cloudy
or is distinctly brown.
Do not use if the outer box or blister seals are broken, or if it has been dropped
with the cap removed.
|
|
Driving or using machines
|
Be careful before you drive or use any machines or tools until you know how XOLAIR
affects you.
|
|
Looking after your medicine
|
Keep it in a refrigerator at 2°C to 8°C. Do not freeze it.
Allow the syringe to reach room temperature (25°C) before injection. If necessary,
XOLAIR may be returned to the refrigerator for later use. The total time that the
syringe is kept at room temperature must not exceed 48 hours.
|
6. Are there any side effects?
All medicines can have side effects although not everybody gets them. The most common
side effects of XOLAIR include fever, injection site reactions, abdominal pain, swelling,
itching, headaches, cold symptoms and upper respiratory infections. XOLAIR may cause
serious allergic reactions.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you
are taking XOLAIR. For more information, including what to do if you have any side effects, see Section
6. Are there any side effects? in the full CMI.
Active ingredient(s):
omalizumab
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using XOLAIR. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using XOLAIR.
Where to find information in this leaflet:
1. Why am I using XOLAIR?
XOLAIR contains the active ingredient omalizumab. XOLAIR is a monoclonal antibody. Monoclonal antibodies are proteins that recognise
and attach specifically to certain proteins in the body. XOLAIR works by blocking
a substance produced by the body called immunoglobulin E (or IgE). IgE contributes
to a type of inflammation that is involved in causing symptoms of conditions such
as:
allergic asthma
chronic rhinosinusitis with nasal polyps (nasal polyps)
chronic spontaneous urticaria (hives)
Allergic asthma
XOLAIR is used to prevent or relieve the symptoms of allergic asthma in people who
are already using preventer puffers containing steroids.
Asthma is a disease where the lining of the lungs becomes inflamed (red and swollen),
making it difficult to breathe. This may be due to a reaction to house dust mites,
smoke or other irritants.
XOLAIR can be used in adults and in children aged 6 years or over in allergic asthma.
Chronic rhinosinusitis with nasal polyps (nasal polyps)
XOLAIR is used to treat a condition called 'chronic rhinosinusitis with nasal polyps'
(nasal polyps) in people whose severe disease is not well controlled with their current
nasal polyps medicines.
XOLAIR helps reduce the size of the polyps and improves symptoms caused by nasal polyps
including nasal congestion, loss of sense of smell, post-nasal drip and runny nose.
This medicine can be used in adults aged 18 years and over in nasal polyps. Its use
in children and adolescents below 18 years of age with nasal polyps has not been studied.
Chronic spontaneous urticaria (hives)
XOLAIR is used to treat a condition called 'chronic spontaneous urticaria' (hives)
in adults and adolescents (12 years of age and older) whose symptoms are not well
controlled by antihistamines. Elderly patients are given the same dosage as younger
adults.
2. What should I know before I use XOLAIR?
Warnings
Do not use XOLAIR if:
you are allergic to omalizumab, or any of the ingredients listed at the end of this
leaflet. Always check the ingredients to make sure you can use this medicine. Some
of the symptoms of an allergic reaction may include:
shortness of breath, wheezing or difficulty breathing or swallowing
swelling of the face, lips, tongue or other parts of the body
rash, itching or hives on the skin
If you think you may be allergic, ask your doctor for advice before using XOLAIR.
Do not take XOLAIR if you have an active infection which your doctor thinks is important.
Do not take XOLAIR if there are visible signs of deterioration. If it has expired
or is damaged, return it to your pharmacist for disposal.
Check with your doctor if you are not sure whether you should start taking this medicine
or you:
have kidney or liver problems
have autoimmune disease
live in a region where parasite infections are frequent, XOLAIR may weaken your resistance
to such infections
had a previous severe allergic reaction (anaphylaxis) resulting, for example, from
medicine, an insect bite or food
had an allergic reaction to latex
have a low platelet count (thrombocytopenia)
You should not use XOLAIR to prevent or treat other allergy-type conditions:
sudden allergic reactions
hyperimmunoglobulin E syndrome (an inherited immunodeficient disorder)
aspergillosis (a fungus-related lung disease)
food allergy, allergic skin rash or hay fever.
You should not use XOLAIR to treat acute asthma symptoms, like a sudden asthma attack.
You will have been given a separate medicine for this.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant. XOLAIR is
not recommended during pregnancy unless the benefits clearly outweigh the potential
risks.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Special populations
XOLAIR is not recommended for children under 6 years of age with allergic asthma because
it has not been studied sufficiently in this age group.
XOLAIR is not recommended for children and adolescents under 18 years of age with
nasal polyps because it has not been studied in this age group.
XOLAIR is not recommended for children under 12 years of age with chronic spontaneous
urticaria because it has not been studied sufficiently in this age group.
XOLAIR may be used by people aged 65 years and over.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
XOLAIR has been used together with inhaled and/or intranasal corticosteroids other
common medicines for asthma/or nasal polyps, as well as H1 or H2 antihistamines and
LTRAs for chronic spontaneous urticaria.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect XOLAIR. Your doctor and pharmacist have more information on medicines to be careful with
or avoid while taking this medicine.
4. How do I use XOLAIR?
How much to give / use
Your doctor will decide how much XOLAIR you need and how often you will need it. This
depends on your body weight and the results of a blood test carried out before the
start of the treatment to measure the amount of IgE in your blood
Allergic asthma and Chronic Rhinosinusitis with Nasal Polyps
You will need 1 to 4 injections at a time. You will need the injections either every
two weeks or every four weeks as prescribed by your doctor.
XOLAIR 300 mg pre-filled syringe and all dose strengths of XOLAIR pre-filled pens
are not intended for use in children under 12 years of age.
XOLAIR 75 mg pre-filled syringe and XOLAIR 150 mg pre-filled syringe or XOLAIR powder
and solvent for solution for injection may be used in children 6 to 11 years of age
with allergic asthma.
Keep taking your current asthma and/or nasal polyps medicine during XOLAIR treatment.
Do not stop taking any asthma and/or nasal polyps medication without talking to your
doctor.
Chronic spontaneous urticaria (hives)
You will need 1 or 2 injections at a time every four weeks.
Keep taking your current medicine for hives during XOLAIR treatment. Do not stop taking
any medicine without talking to your doctor.
Do not exceed the recommended dose.
Follow the instructions provided and use XOLAIR until your doctor tells you to stop.
How to give XOLAIR
Follow all directions given to you by your doctor, nurse or pharmacist carefully.
They may differ from the information contained in this leaflet.
Always use XOLAIR as your doctor has told you. You should check with your doctor,
nurse or pharmacist if you are not sure.
XOLAIR is intended for subcutaneous use. This means that it is injected into the fatty
tissue just under the skin.
There are two different ways XOLAIR can be given:
pre-filled syringe and pre-filled pen: designed to be administered by patients or
carers or healthcare professionals.
powder for injection vial with diluent: for use by healthcare professionals only.
You and your doctor should decide if you should inject XOLAIR yourself.
After proper training in subcutaneous injection technique, patients ≥ 12 years old
may self-inject XOLAIR or, for all patients ≥ 6 years old, the injection may be given
by a caregiver if your doctor determines that it is appropriate.
It is important not to try to inject yourself until you have been trained by your
doctor, nurse or pharmacist. A caregiver may also give you your XOLAIR injection after
proper training.
If you do not understand the instructions on the label ask your doctor or pharmacist
for help.
Choosing the injection sites
The injection sites are where the skin will be pierced to administer the subcutaneous
injection.
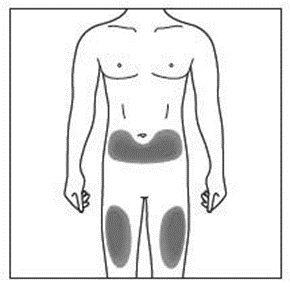
The recommended site is the front of your thighs. You may also use the lower abdomen,
but not the area five centimetres around the navel (belly button). Choose a different site
each time you give yourself an injection.
If a caregiver is giving you the injection, the outer upper arms may also be used
(Shown in grey in the diagram for illustrative purposes).
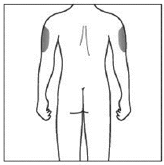
Do not inject into areas where the skin is tender, bruised, red or hard. Avoid areas
with scars or stretch marks.

For detailed instructions on how to inject XOLAIR, see Instructions for Use at the
end of this leaflet:
Read the Instructions for Use on how to use XOLAIR all the way through before removing
XOLAIR end from the refrigerator. These instructions are to help you to inject correctly.
How long to use XOLAIR
Keep using this medicine for as long as your doctor tells you.
Your doctor will regularly monitor your condition to check that the treatment is having
the desired effect.
If you forget to use XOLAIR
If you have missed an appointment to get a XOLAIR injection, contact your doctor and
get it as soon as you remember.
If you have forgotten to inject a dose of XOLAIR, inject the next dose as soon as
you remember. Then talk to your doctor to discuss when you should inject the next
dose.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to have your medicine, ask your pharmacist for some
hints.
Do not take a double dose to make up for the dose you missed.
If you use too much XOLAIR
If you think that you have used too much XOLAIR, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(by calling 13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using XOLAIR?
Things you should do
Discontinue treatment and tell your doctor or pharmacist immediately if you get any
of these symptoms during treatment with XOLAIR.
Call your doctor straight away if you have:
Signs or symptoms of a potentially serious infection. These may include:
fever, flu-like symptoms, night sweats
feeling tired or short of breath; cough which will not go away
warm, red and painful skin, or a painful skin rash with blisters
burning when passing urine
Signs or symptoms of an allergic reaction. These may include:
difficulty breathing or swallowing
low blood pressure, which can cause dizziness or light-headedness
swelling of the face, lips, mouth or throat
severe itching of the skin, with a red rash or raised bumps
A specific type of allergic reaction (serum sickness) has also been observed in patients
treated with XOLAIR. Signs may include:
joint pain
stiffness
rash
fever
swollen/enlarged lymph nodes
Keep all of your doctor's appointments so that your progress can be checked. Your doctor will do tests from time to time to make sure the medicine is working
and to prevent unwanted side effects. Your doctor will decide if and when you may
restart the treatment.
If you need to be vaccinated, tell your doctor you are taking XOLAIR before you have
the vaccination.
If you are about to be started on any new medicine, remind your doctor and pharmacist
that you are taking XOLAIR.
Remind any doctor, dentist or pharmacist you visit that you are using XOLAIR.
Things you should not do
Do not stop taking your medicine or change dosage without checking with your doctor
first.
Never leave the prefilled syringe or prefilled pen lying around where others might
tamper with it.
Do not open the sealed box until you are ready to use XOLAIR.
Do not use this medicine if the liquid contains easily visible particles, is cloudy
or is distinctly brown.
Do not use the prefilled syringe or prefilled pen if either the seal on the outer
box or the seal of the blister are broken. It may not be safe for you to use.
Do not shake the prefilled syringe or the prefilled pen.
Do not use the pen if it has been dropped with the cap removed.
Do not take it to treat any other complaints unless your doctor tells you to.
Do not give this medicine to anyone else, even if they have the same condition as
you.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how XOLAIR
affects you.
No studies on the effects on the ability to drive and use of machines have been performed
on XOLAIR.
Looking after your medicine
Keep it in a refrigerator at 2°C to 8°C. Do not freeze it.
Store it in the carton in order to protect it from light. Follow the instructions
in the carton on how to take care of your medicine properly.
Prior to use, allow the syringe to reach room temperature (25°C) before injection.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do
not store it:
in the bathroom or near a sink, or
in the car or on window sills.
A locked section of the refrigerator, at least one-and-a-half metres above the ground,
is a good place to store XOLAIR.
Keep it where young children cannot reach it.
When to discard your medicine
If necessary, the product may be returned to the refrigerator for later use. The total
time that the syringe is kept at room temperature must not exceed 48 hours.
Discard any unused product.
Dispose of the used XOLAIR prefilled syringe or prefilled pen immediately after use
in a sharps container. The XOLAIR prefilled syringe or prefilled pen should never be re-used.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy
for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects although not everybody gets them. If you do experience
any side effects, most of them are minor and temporary. However, some side effects
may need medical attention.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you
are taking XOLAIR.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Less serious side effects
|
Less serious side effects
|
What to do
|
|
bruising, redness or pain at the injection site
mild skin rash
headache
tiredness
hair loss
joint swelling
fever (very common in children)
sore throat and blocked nose (nasopharyngitis)
feeling of pressure or pain in the cheeks and forehead (sinusitis and sinus headache)
upper respiratory tract infection, such as inflammation of the pharynx and common
cold
burning sensation or pain when passing urine and having to urinate frequently (possible
symptom of an urinary tract infection)
pain in upper or lower limbs (arms and/or legs)
pain in the upper part of the abdomen
pain in muscles and/or bones and/or joints (musculoskeletal pain, myalgia, arthralgia)
|
Speak to your doctor if you have any of these less serious side effects and they worry
you.
|
Serious side effects
|
Serious side effects
|
What to do
|
|
Allergy related
anaphylactic shock, where signs may include: swelling of the throat and mouth, difficulty
breathing, lightheadedness, confusion, blue skin or lips, collapsing and losing consciousness
serum sickness, where signs may include joint pain, stiffness, rash, fever, swollen/enlarged
lymph nodes
Blood related
abnormal bleeding or bruising
General (Churg-Strauss syndrome)
pain, numbness or tingling in the arms and legs, lumps or raised patches in the skin,
weakness and fatigue, loss of appetite, and weight loss
|
Call your doctor straight away, or go straight to the Emergency Department at your
nearest hospital if you notice any of these serious side effects.
|
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What XOLAIR contains
|
Active ingredient
|
omalizumab
|
|
Other ingredients
|
Solution for injection in prefilled syringe and prefilled pen:
arginine hydrochloride
histidine
histidine hydrochloride monohydrate
polysorbate 20
water for injections
Vial powder for injection:
sucrose
histidine
histidine hydrochloride monohydrate
polysorbate 20
|
|
Potential allergens
|
Sucrose (powder for injection)
|
Do not take this medicine if you are allergic to any of these ingredients.
What XOLAIR looks like
XOLAIR solution for injection in pre-filled syringe and pre-filled pen is a clear
liquid. Its colour may vary from colourless to pale brownish-yellow.
XOLAIR powder for injection vial with diluent is a white to off-white powder that
is reconstituted with water for injection.
XOLAIR solution for injection in pre-filled syringe is available in 75 mg/0.5 mL (AUST
R 201124), 150 mg/1 mL (AUST R 201126) or 300 mg/2mL (AUST R 429450).
Available as a single packaged and in multipacks containing either 4 or 10 individually
packaged pre-filled syringes.
XOLAIR solution for injection in pre-filled autoinjector pen is available in 75 mg/0.5
mL (AUST R 429510), 150 mg/1 mL (AUST R 429511) or 300 mg/2mL (AUST R 429512).
XOLAIR powder for injection vial and diluent is available as a pack containing 1 single-use
vial of either 75 mg (AUST R 115399) or 150 mg (AUST R 82744) powder and 1 ampoule
of water for injections for use as diluent.
Who distributes XOLAIR
Novartis Pharmaceuticals Australia Pty Limited
ABN 18 004 244 160
54 Waterloo Road
Macquarie Park NSW 2113
Telephone 1 800 671 203
® = Registered Trademark
This leaflet was prepared in September 2024.
(xol300824c_v3 based on PI xol300824i)
8. Instructions for use of XOLAIR pre-filled syringe (pre-filled syringe with 26 gauge
staked needle)
Read ALL the way through these instructions before injecting. If your doctor decides
that you or a caregiver may be able to give your injections of Xolair at home, you
need to be trained by your doctor, nurse or pharmacist before you inject yourself
or others. Children (6 to less than 12 years of age) are not expected to inject Xolair
themselves, however, if deemed appropriate by their doctor, a caregiver may give them
their Xolair injection after proper training. The box contains a Xolair pre-filled
syringe individually sealed in a plastic tray.
Xolair pre-filled syringe is available in two strengths, 75 mg and 150 mg. You may
receive one or both strengths from the pharmacy.
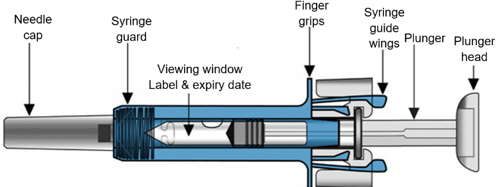
Your Xolair 75 mg pre-filled syringe (pre-filled syringe with 26-gauge staked needle)
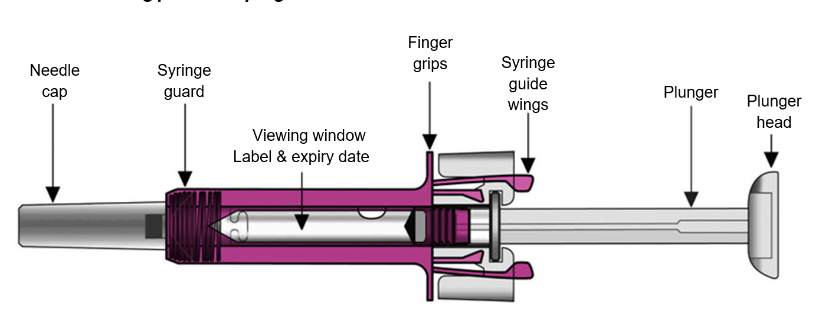
Your Xolair 150 mg pre-filled syringe
After the medicine has been injected the syringe guard will be activated to cover
the needle. This is intended to protect against accidental needle stick injuries.
Other items you need for your injection:
Alcohol swab.
Cotton ball or gauze.
Sharps disposal container
Adhesive plaster
Important safety information
Caution: Keep the syringe out of the sight and reach of children.
The needle cap of the syringe may contain dry rubber (latex), which should not be
handled by anyone who is sensitive to this substance.
Do not open the sealed outer box until you are ready to use this medicine.
Do not use this medicine if either the seal on the outer box or the seal of the plastic
tray is broken, as it may not be safe for you to use.
Do not use if the syringe has been dropped onto a hard surface or dropped after removing
the needle cap.
Never leave the syringe where others might tamper with it.
Do not shake the syringe.
Be careful not to touch the syringe guard wings before use. If the wings are touched,
the syringe guard may be activated too early.
Do not remove the needle cap until just before you give the injection.
The syringe cannot be re-used. Dispose of the used syringe immediately after use in
a sharps container.
Storage of the Xolair pre-filled syringe
Store this medicine sealed in its outer box to protect it from light. Store in the
refrigerator between 2°C and 8°C. DO NOT FREEZE.
Prior to use, allow the syringe to reach room temperature (25°C) before preparing
it for injection (it will take about 30 minutes). If necessary, the product may be
returned to the refrigerator for later use. The total time that the syringe is kept
at room temperature must not exceed 48 hours.
Do not try to warm the syringe using an external heat source.
Do not use the syringe after the expiry date which is stated on the outer box and
syringe label. If it has expired, return the entire pack to the pharmacy.
The injection site
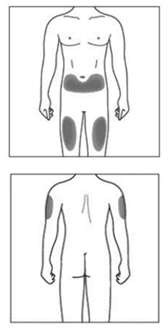
The injection site is the place on the body where you are going to use the syringe.
The recommended site is the front of the thighs. You may also use the lower abdomen,
but not the area 5 centimeters around the navel (belly button).
If you need to give more than one injection for the full dose, choose a different
injection site each time you inject.
If injecting more than once into the same area, ensure a distance of at least 4 cm
between injection sites.
Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid areas
with scars or stretch marks.
If a caregiver is giving the injection, the outer upper arms may also be used.
Preparing the Xolair pre-filled syringe for use
Note: Depending on the dose prescribed to you by your doctor, you may need to prepare one
or more pre-filled syringes, and inject the contents of them all.
The following table give EXAMPLES of how many injections of each strength you need
for a given dose. Please discuss with your doctor how frequent (4 weekly or 2 weekly)
you need to administer each dose
1. Take the box containing the syringe out of the refrigerator and leave it unopened for about 30 minutes so that it reaches room temperature (leave the syringe in the
box to protect it from light).
2. When you are ready to use the syringe, wash your hands thoroughly with soap and water.
3. Clean the injection site with an alcohol swab.
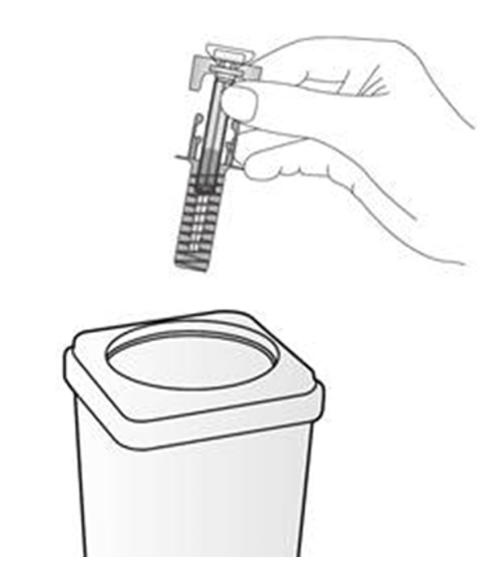
4. Remove the plastic tray from the box and peel back the paper cover. Gripping the middle
of the syringe guard, lift the syringe out of the tray.
5. Inspect the syringe. The liquid should be clear to slightly cloudy. Its colour may
vary from colourless to pale brownish-yellow. You may see an air bubble, which is
normal. DO NOT USE if the syringe is broken or if the liquid looks distinctly cloudy
or distinctly brown, or contains particles. In all these cases, return the entire
pack to the pharmacy.
6. Holding the syringe horizontally, look into the viewing window to check the expiry
date printed on the label. (Note: It is possible to rotate the inner part of the syringe
assembly so that the label can be read in the viewing window. DO NOT USE if the product
has expired. If expired, return the entire pack to the pharmacy.)
How to use the Xolair pre-filled syringe
Carefully remove the needle cap from the syringe. Discard the needle cap. You may
see a drop of liquid at the end of the needle. This is normal.
Gently pinch the skin at the injection site and insert the needle as shown. Push the
needle all the way in to ensure that the medicine can be fully administered.
Hold the syringe as shown. Slowly depress the plunger as far as it will go so that the plunger head is completely between the syringe guard wings.
Keep the plunger fully depressed while you carefully lift the needle straight out from the injection site.
Slowly release the plunger and allow the syringe guard to automatically cover the
exposed needle.
There may be a small amount of blood at the injection site. You can press a cotton
ball or gauze over the injection site and hold it for 30 seconds. Do not rub the injection
site. You may cover the injection site with a small adhesive bandage, if needed.
Disposal instructions
Dispose of the used syringe immediately in a sharps container (closable, puncture
resistant container). For the safety and health of you and others, needles and used
syringes must never be re-used. Any unused medicinal product or waste material should be disposed of
in accordance with local requirements. Do not throw away any medicines via wastewater
or household waste. Ask your pharmacist how to throw away medicines you no longer
use. These measures will help protect the environment.
9. Instructions for use of XOLAIR pre-filled syringe (pre-filled syringe with 27-gauge
staked needle)
This “Instructions for Use” contains information on how to inject Xolair.
If your doctor decides that you or your caregiver may be able to give your injections
of Xolair at home, ensure that your doctor or nurse shows you or your caregiver how
to prepare and inject with the Xolair pre-filled syringe before you use it for the
first time.
Children below 12 years of age are not expected to inject Xolair themselves, however,
if deemed appropriate by their doctor, a caregiver may give them their Xolair injections
after proper training.
Be sure that you read and understand this “Instructions for Use” before injecting
with the Xolair pre-filled syringe. Talk to your doctor if you have any questions.
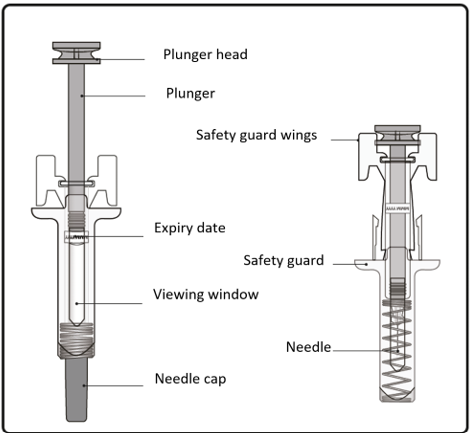
Important information you need to know before injecting Xolair
Xolair is for subcutaneous injection only (inject directly into fatty layer under
the skin).
Do not use the pre-filled syringe if either the seal on the outer carton or the seal of
the plastic tray is broken.
Do not use if the pre-filled syringe has been dropped onto a hard surface or dropped after
removing the needle cap.
Do not inject if the pre-filled syringe has been kept out of the refrigerator for more than
a total of 48 hours. Dispose of it (see Step 12) and use a new pre-filled syringe
for your injection.
The pre-filled syringe has a safety guard that will be activated to cover the needle
after the injection is finished. The safety guard will help to prevent needlestick
injuries to anyone who handles the pre-filled syringe after injection.
Do not try to re-use or take apart the pre-filled syringe.
Do not pull back on the plunger.
Store Xolair
Store in a refrigerator (2°C to 8°C). The carton containing the pre-filled syringe
can be stored for a total time of 48 hours at room temperature (25°C) before use.
It can be placed back in the refrigerator if necessary.
Do not freeze.
Keep the pre-filled syringe in the original carton until ready to use in order to
protect from light.
Keep the pre-filled syringe out of sight and reach of children.
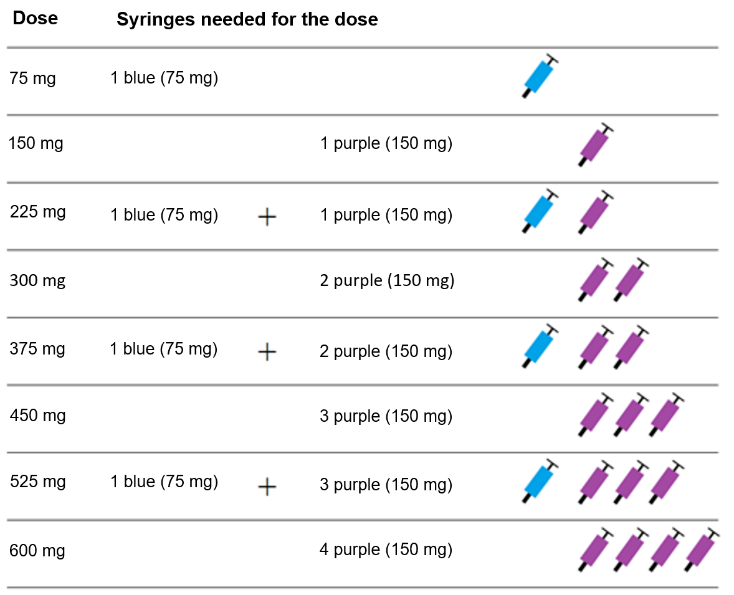
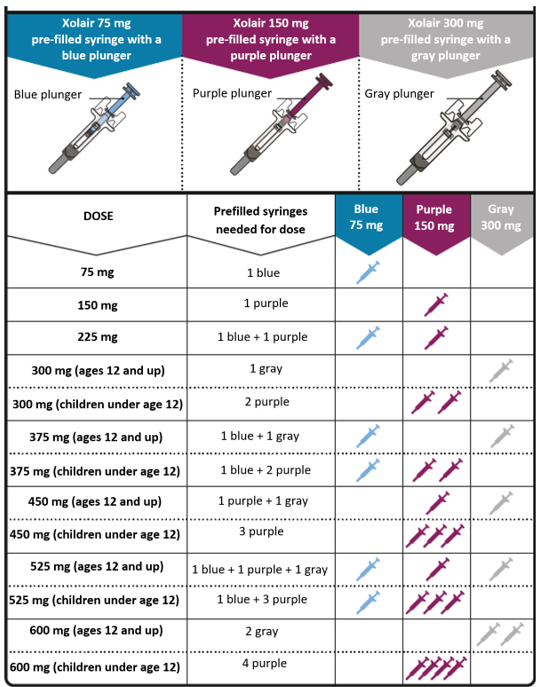
Dosing table
Xolair pre-filled syringes are available in 3 dose strengths (one pre-filled syringe
in each carton). These instructions are to be used for all 3 dose strengths.
Depending on the dose prescribed to you by your doctor, you may need to select one
or more pre-filled syringes, and inject the contents of them all in order to deliver
your full dose. The Dosing Table below shows the combination of pre-filled syringes
needed to deliver your full dose.

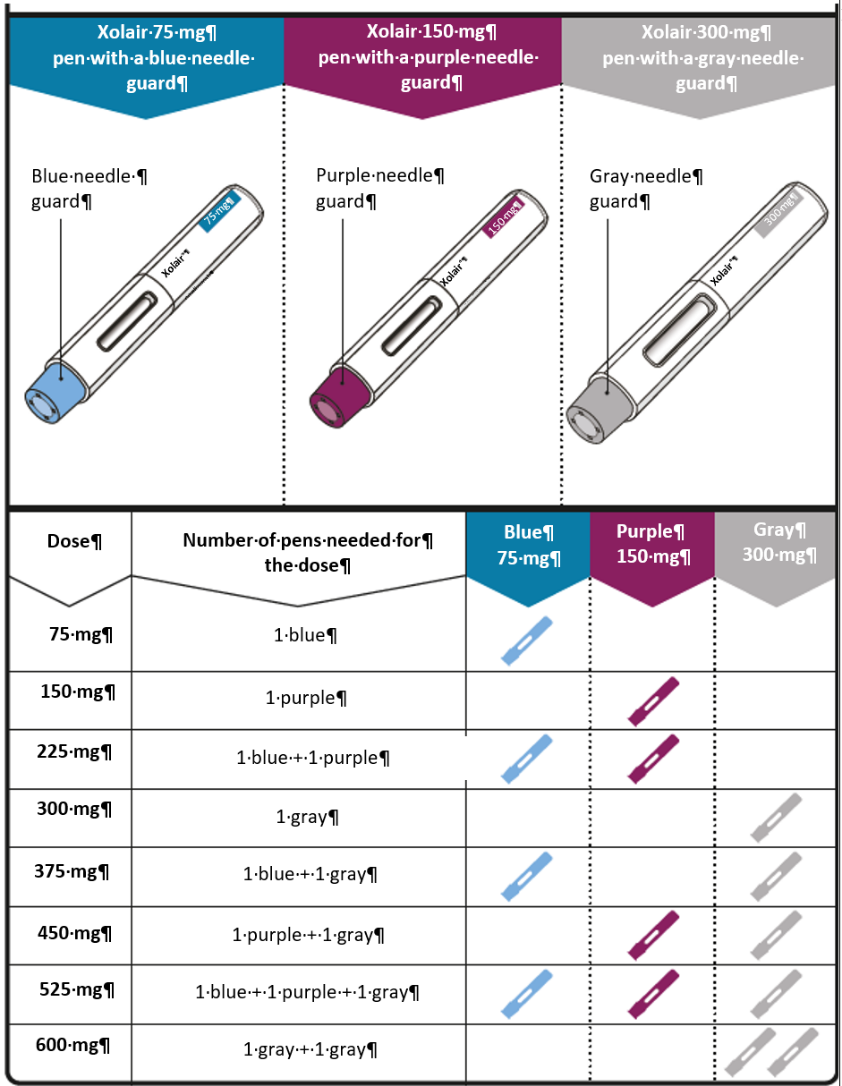
Important: If the dose is for a child under age 12 it is recommended to use only blue (75 mg)
and purple (150 mg) pre-filled syringes. Refer to the Dosing Table below for the recommended
combination of pre-filled syringes for children under age 12.
Contact your doctor if you have questions on the Dosing Table.
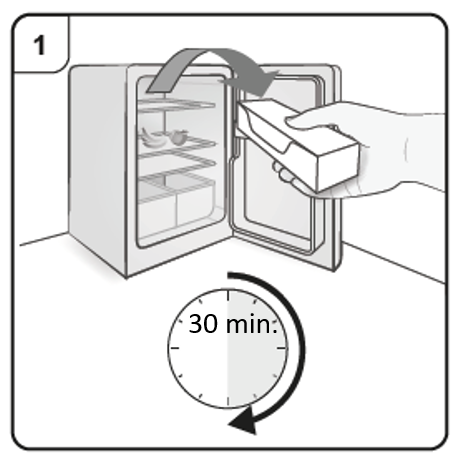
Prepare to inject Xolair
Step 1. Bring to room temperature
Take the carton containing the pre-filled syringe out of the refrigerator and leave it unopened so that it reaches room temperature (minimum 30 minutes).
Note: If you need more than one pre-filled syringe (one pre-filled syringe per carton)
to deliver your full dose (see Dosing Table), take all the cartons out of the refrigerator
at the same time.
Step 2. Gather supplies
You will need the following supplies (not included in the carton):
Alcohol wipe
Cotton ball or gauze pad
Sharps disposal container
Adhesive plaster
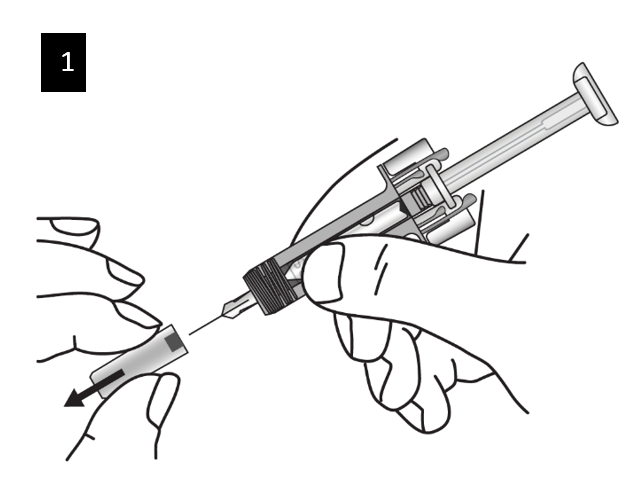
Step 3. Unpack
Open the plastic tray by peeling away the cover. Remove the pre-filled syringe by
holding it in the middle as shown.
Do not remove the needle cap until you are ready to inject.
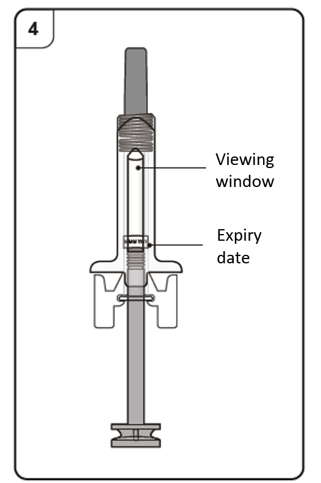
Step 4. Inspect the pre-filled syringe
Look through the viewing window of the pre-filled syringe.
The liquid inside should be clear to slightly cloudy. Its color may vary from colorless
to pale brownish-yellow. You may see air bubbles in the liquid, which is normal. Do not try to remove the air.
Do not use the pre-filled syringe if the liquid contains particles, or if the liquid looks
distinctly cloudy or distinctly brown.
Do not use the pre-filled syringe if it looks damaged or if it has leaked.
Do not use the pre-filled syringe after the expiry date (EXP), which is printed on the pre-filled
syringe label and carton.
In all of these cases, contact your doctor, nurse or pharmacist.
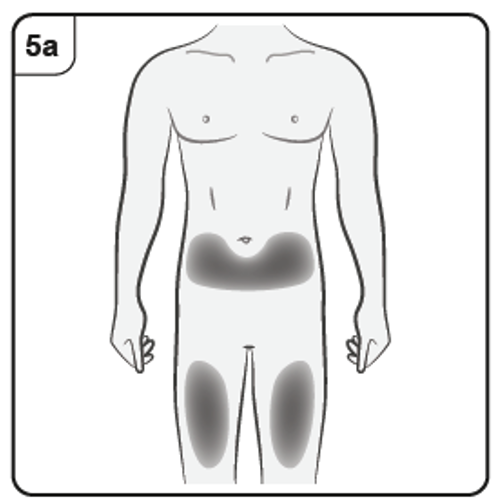
Step 5. Choose injection site
You should inject into the front of the thighs or the lower stomach area but not the
area 5 cm around the belly button.
Do not inject into skin that is tender, bruised, red, scaly or hard or into areas with scars
or stretch marks.
Note: If you need more than one pre-filled syringe to deliver your full dose, make
sure your injections are at least 2 cm apart.
If your caregiver, doctor or nurse is giving you the injection, they may also inject
into the outer upper arm.
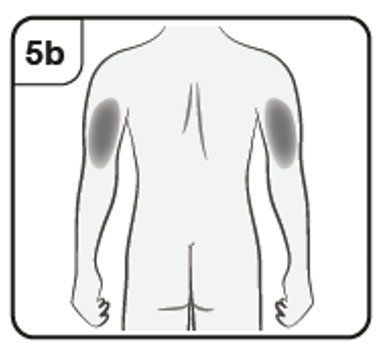
Inject with Xolair
Step 6. Clean injection site
Clean your hands.
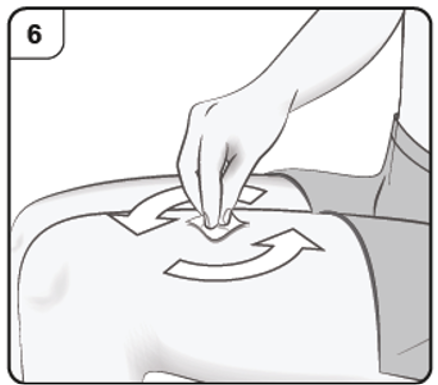
Clean the chosen injection site with an alcohol wipe. Leave it to dry before injecting.
Do not touch or blow on the cleaned skin before injecting.
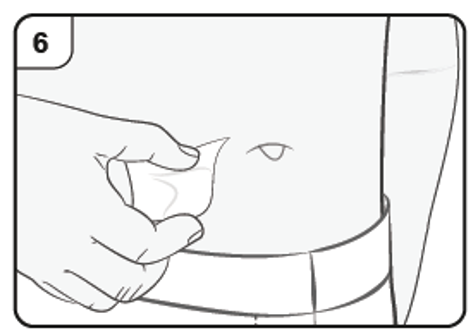
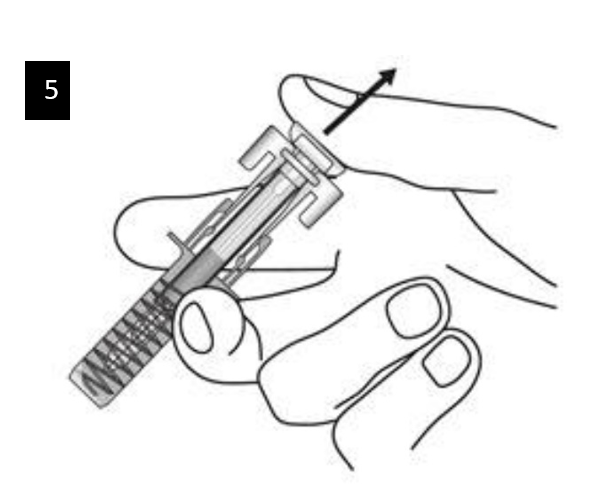
Step 7. Remove needle cap
Firmly pull straight to remove the needle cap from the pre-filled syringe. You may
see a drop of liquid at the end of the needle. This is normal.
Do not put the needle cap back on.
Throw away the needle cap.
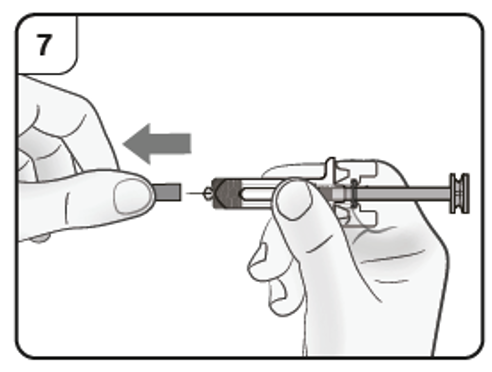
Step 8. Insert needle
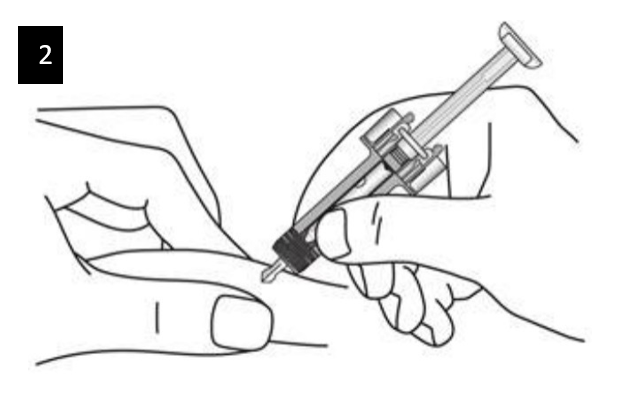
Gently pinch the skin at the injection site and hold the pinch throughout the injection.
With the other hand insert the needle into the skin at an angle of approximately 45
degrees as shown.
Do not press the plunger while inserting the needle.
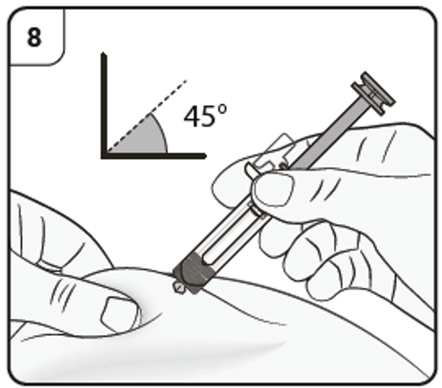
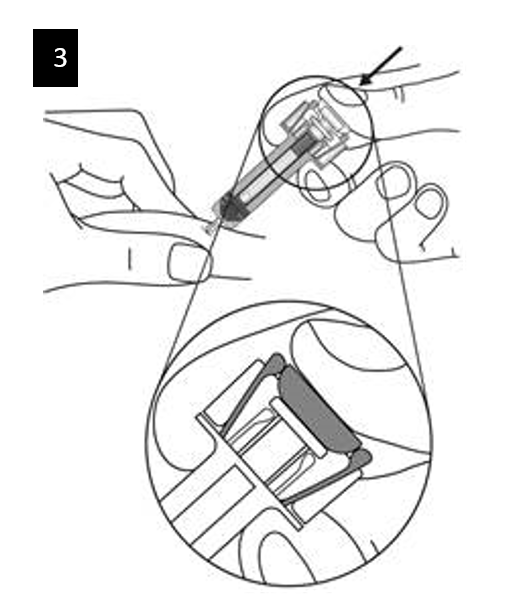
Step 9. Start injection
Continue to pinch the skin. Slowly press the plunger as far as it will go. This will ensure that a full dose is injected.
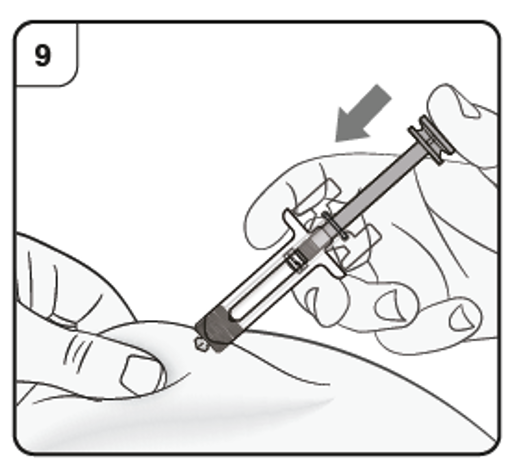
Step 10. Complete injection
Confirm that the plunger head is between the safety guard wings as shown. This will
ensure that the safety guard has been activated and will cover the needle after the
injection is finished.
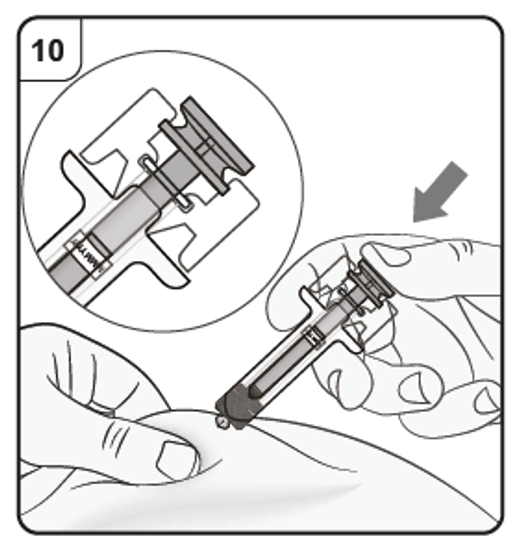
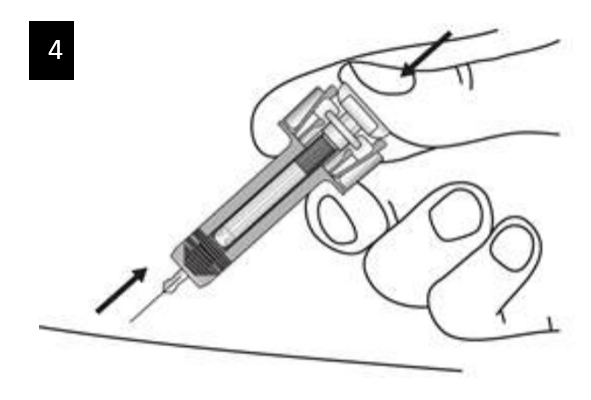
Step 11. Release plunger
Keeping the pre-filled syringe at the injection site, slowly release the plunger until
the needle is covered by the safety guard. Remove the pre-filled syringe from the
injection site and release the pinch.
There may be a small amount of blood at the injection site. You can press a cotton
ball or gauze pad over the injection site until any bleeding stops. Do not rub the injection site. If needed, cover the injection site with a small adhesive
plaster.
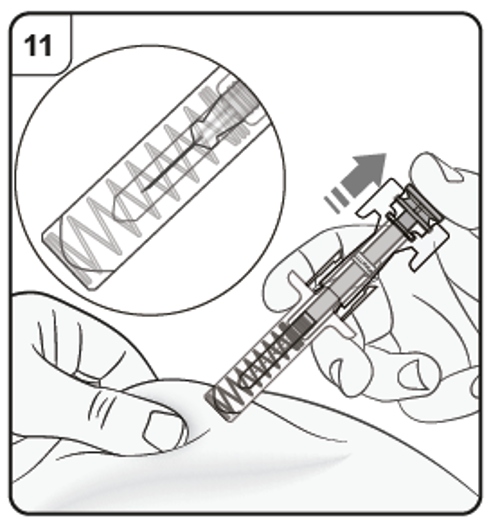
Note: If you need more than one pre-filled syringe to deliver your full dose, throw
away the used pre-filled syringe as described in Step 12.
Repeat Step 2 to Step 12 again for all the pre-filled syringes needed to deliver your
full dose.
Carry out the injections immediately one after another.
Make sure the injections are at least 2 cm apart.
After the injection
Step 12. Dispose of the pre-filled syringe
Put the used pre-filled syringe in a sharps disposal container (i.e. a puncture-resistant
closable container, or similar) immediately after use.
Do not throw away the pre-filled syringe into household waste.
Do not try to put the needle cap back onto the syringe.
Talk to your doctor or pharmacist about proper disposal of the sharps disposal container.
There may be local regulations for disposal.
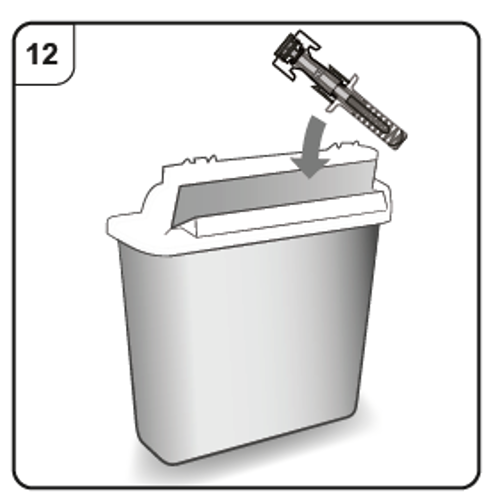
10. Instructions for use of XOLAIR pre-filled pen
This “Instructions for Use” contains information on how to inject Xolair.
If your doctor decides that you or your caregiver may be able to give your injections
of Xolair at home, ensure that your doctor or nurse shows you or your caregiver how
to prepare and inject with the Xolair pen before you use it for the first time.
This Xolair pen is intended to be used for patients ages 12 and up.
Be sure that you read and understand this “Instructions for Use” before injecting
with the Xolair pen. Talk to your doctor if you have any questions.
Important information you need to know before injecting Xolair
Xolair is for subcutaneous injection only (inject directly into fatty layer under
the skin).
Do not use the pen if the seal on the outer carton is broken.
Do not use if the pen has been dropped after removing the cap.
Do not inject if the pen has been kept out of the refrigerator for more than a total
of 48 hours. Dispose of it (see Step 13) and use a new pen for your injection.
Do not touch or push the needle guard as you could get injured. Touching or pushing on the
needle guard could cause a needlestick injury.
Do not try to re-use or take apart the pen.
Do not try to reattach the cap once it has been taken off.
Store Xolair
Store in a refrigerator (2°C and 8°C). The carton containing the pen can be stored
for a total time of 48 hours at room temperature (25°C) before use. It can be placed
back in the refrigerator if necessary.
Do not freeze.
Keep the pen in the original carton until ready to use in order to protect from light.
Keep the pen out of sight and reach of children.
Dosing table
Xolair pens are available in 3 dose strengths (one pen in each carton). These instructions
are to be used for all 3 dose strengths.
Depending on the dose prescribed to you by your doctor you may need to select one
or more pens, and inject the contents of them all in order to deliver your full dose.
The Dosing Table below shows the combination of pens needed to deliver your full dose.
Contact your doctor if you have questions on the Dosing Table.
Prepare to inject Xolair
Step 1. Bring to room temperature
Take the carton containing the pen out of the refrigerator and leave it unopened so that it reaches room temperature (minimum 30 minutes).
Note: If you need more than one pen (one pen in each carton) to deliver your full
dose (see Dosing Table), take all the cartons out of the refrigerator at the same
time.
Step 2. Gather supplies
You will need the following supplies (not included in the carton):
Alcohol wipe
Cotton ball or gauze pad
Sharps disposal container
Adhesive plaster
Step 3. Unpack
Take the pen out of the outer carton.
Do not remove the cap until you are ready to inject.
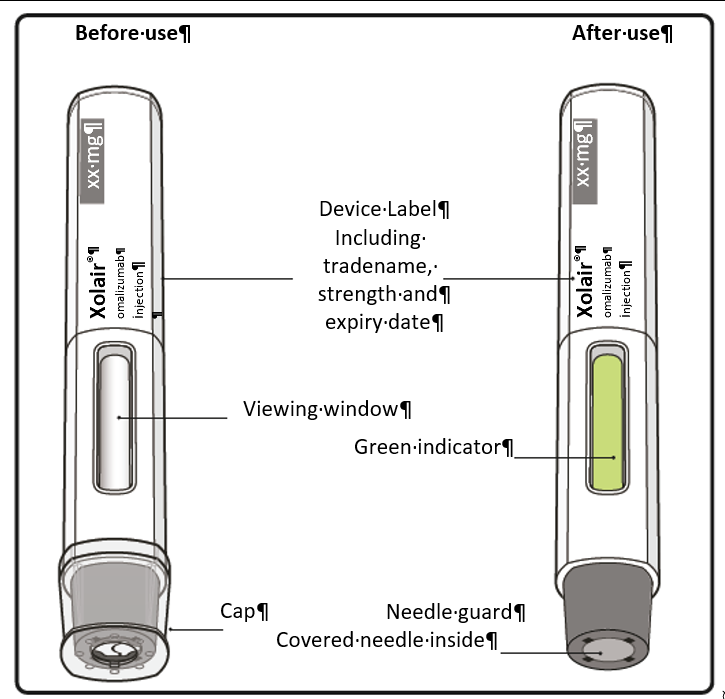
Step 4. Inspect the pen
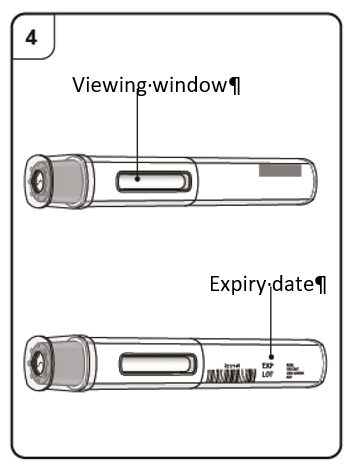
Look through the viewing window of the pen.
The liquid inside should be clear to slightly cloudy. Its color may vary from colorless
to pale brownish-yellow. You may see air bubbles in the liquid, which is normal.
Do not use the pen if the liquid contains particles, or if the liquid looks distinctly cloudy
or distinctly brown.
Do not use the pen if it looks damaged.
Do not use the pen after the expiry date (EXP), which is printed on the pen label and carton.
In all of these cases, contact your doctor, nurse or pharmacist.
Step 5. Choose injection site
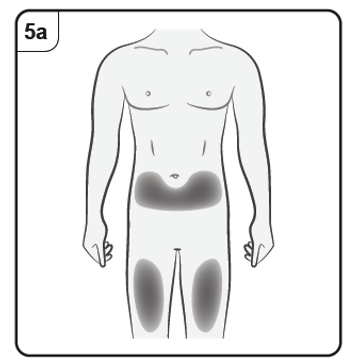
You should inject into the front of the thighs or the lower stomach area but not the
area 5 cm around the belly button.
Do not inject into skin that is tender, bruised, red, scaly or hard or into areas with scars
or stretch marks.
Note: If you need more than one pen to deliver your full dose, make sure your injections
are at least 2 cm apart.
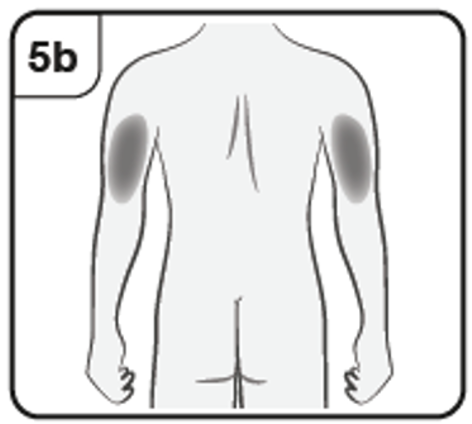
If a caregiver, doctor or nurse is giving the injection, they may also inject into
the outer upper arm.
Inject with Xolair
Step 6. Clean injection site
Clean your hands.
Clean the chosen injection site with an alcohol wipe. Leave it to dry before injecting.
Do not touch or blow on the cleaned skin before injecting.
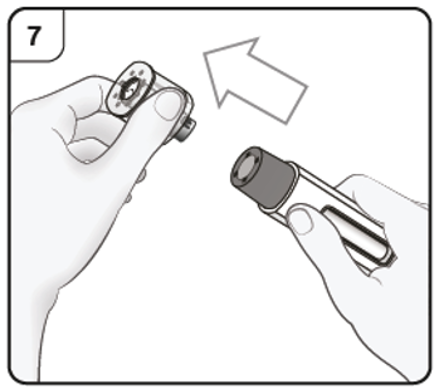
Step 7. Remove cap
Pull the cap straight off in the direction of the arrow.
Do not put the cap back on. Throw away the cap.
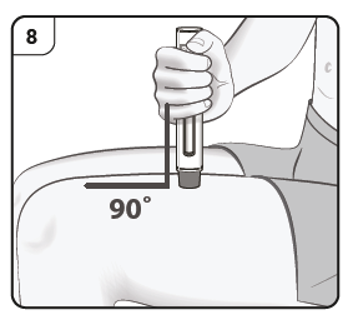
Step 8. Position the pen
Hold the pen comfortably with the needle guard directly against the skin.
The pen should be at a 90° angle to the skin as shown.
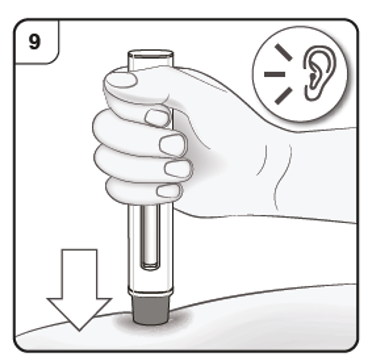
Step 9. Start injection
Push and hold the pen firmly against the skin. Listen for the 1st click which indicates that the injection has started.
Listen for ‘click’ sound.
Step 10. Monitor injection
Keep holding the pen firmly down against the skin. The green indicator shows the progress
of the injection.
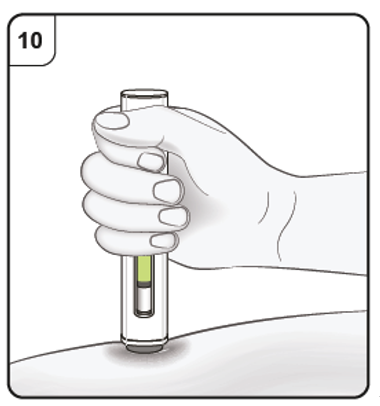
Step 11. Complete injection
Listen for the 2nd click. This indicates the injection is almost complete.
Keep holding the pen in position until the green indicator has stopped moving to make
sure the injection is complete. Remove the pen from the skin. The needle is automatically covered by the needle guard.
The injection is now complete.
Listen for ‘click’ sound.
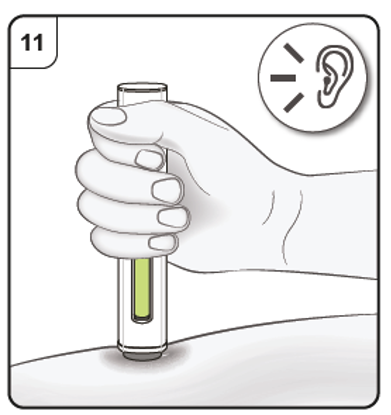
After the injection
Step 12. Check green indicator
If the green indicator has not completely filled the viewing window, contact your
doctor or nurse.
There may be a small amount of blood at the injection site.
You can press a cotton ball or gauze pad over the injection site until any bleeding
stops.
Do not rub the injection site. If needed, cover your injection site with a small adhesive
plaster.
Note: If you need more than one pen to deliver your full dose, throw away the used
pen as described in Step 13.
Repeat Step 2 to Step 13 again for all the pens needed to deliver your full dose.
Carry out the injections immediately one after another.
Make sure the injections are at least 2 cm apart.
Step 13. Dispose of the pen
Put the used pen in a sharps disposal container (i.e. a puncture-resistant closable
container, or similar) right away after use.
Do not throw away (dispose of) the pen into household waste.
Talk to your doctor or pharmacist about proper disposal of the sharps disposal container.
There may be local regulations for disposal.
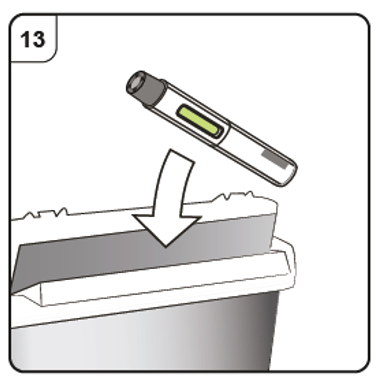
11. Information for the Healthcare Professional: XOLAIR powder vial & diluent
**The following information is intended for MEDICAL or HEALTHCARE PROFESSIONALS only.**
Xolair is for single use in one patient only and contains no antimicrobial agent.
To reduce microbiological hazard, the product should be used immediately after reconstitution.
The lyophilized product takes 15 to 20 minutes to dissolve, although in some cases
it may take longer. The fully reconstituted product will appear clear to slightly
opalescent, colourless to pale brownish-yellow and may have a few small bubbles or
foam around the edge of the vial. Because the reconstituted product is somewhat viscous,
care must be taken to WITHDRAW ALL OF THE PRODUCT from the vial before expelling any
air or excess solution from the syringe in order to obtain the full 1.2mL dose.
To prepare Xolair 150 mg Powder Vials for subcutaneous administration, please adhere
to the following instructions:
1. Draw 1.4 mL of water for injections from the ampoule into a 3 mL syringe equipped
with a 1 inch, large bore 18-gauge needle.
2. With the vial placed upright on a flat surface, insert the needle and transfer
the water for injections into the omalizumab vial using standard aseptic techniques,
directing the water for injections directly onto the powder.
3. Keeping the vial in the upright position, vigorously swirl the vial (do not shake)
for approximately 1 minute to evenly wet the powder.
4. To aid dissolution after completing step 3, gently swirl the vial for 5 to 10 seconds
approximately every 5 minutes in order to dissolve any remaining solids.
Note: The powder typically takes 15 to 20 minutes to dissolve completely, although it may take longer. If this is the case, repeat
step 4 until there are no visible gel-like particles in the solution.
When the medicinal product is fully dissolved, there should be no visible gel-like
particles in the solution. Small bubbles or foam around the edge of the vial are common.
The reconstituted medicinal product will appear clear to slightly opalescent, colourless
to pale brownish-yellow. Do not use if solid particles are present.
5. Invert the vial for at least 15 seconds in order to allow the solution to drain
towards the stopper. Using a new 3 mL syringe equipped with a 1 inch, large bore,
18-gauge needle, insert the needle into the inverted vial. Keeping the vial inverted,
position the needle tip at the very bottom of the solution in the vial when drawing
the solution into the syringe. Before removing the needle from the vial, pull the
plunger all the way back to the end of the syringe barrel in order to remove all of the solution from the inverted vial.
6. Replace the 18-gauge needle with a 25-gauge needle for subcutaneous injection.
7. Expel air, large bubbles and any excess solution in order to obtain the required
1.2 mL dose. A thin layer of small bubbles may remain at the top of the solution in
the syringe. Because the solution is slightly viscous, the injection may take 5 to
10 seconds to administer the solution by subcutaneous injection.
The vial delivers 1.2 mL (150 mg) of Xolair.
8. The injections are administered subcutaneously in the deltoid region of the arm,
the lower abdomen (but not the area 5 centimeters around the navel) or the thigh.