At-home test kits: history, types, and general working principles
Developmental regulations and availability of at-home test kits
Benefits and ends of at-home test kits
What measures can you follow to get reliable results?
References
Further reading
At-home test kits, also known as at-home medical tests or self-tests, are kits that you can purchase online, at supermarkets, or at your local pharmacy and use to detect or monitor certain diseases and conditions in the privacy of your home.1
Some common at-home test kits are blood glucose tests, which help you to manage diabetes, genetic tests to determine whether you are at a high risk of certain diseases, and pregnancy tests.

Image Credit: Southworks/Shutterstock.com
At-home test kits: history, types, and general working principles
In the mid-1970s, the first at-home pregnancy test kits were developed by Margaret M. Crane for pregnancy testing, which helped determine whether a woman had conceived or not in the privacy of their own home.2 Over time, medical and technological evolution has enabled the development of more sensitive and accurate at-home test kits.
There are two types of at-home tests, namely, self-tests and self-collection tests. Self-test kits require you to collect samples, such as urine, blood, or saliva, and apply them to the kit as directed. These tests generate immediate results.
Self-collection test kits allow you to collect samples at home, which are then packed and posted in the laboratory for analysis. After evaluation, your test results are sent via email, post, or directly to your healthcare providers.
Several at-home tests are developed based on lateral flow assays (LFA), which determine and quantify a wide range of biomarkers.3 LFA offers a paper-based platform that can analyze samples within 5 to 30 minutes.

Image Credit: Ground Picture/Shutterstock.com
The main principle of LFA involves a liquid sample (e.g., saliva, urine, or blood) containing the biomarker moving through a polymeric lateral flow test strip via capillary action. The molecules of polymeric strips interact with the analyte, and this interaction (e.g., an antigen-antibody reaction) is detected through a simple detection system.
Based on the working principle, LFA has been categorized into lateral flow immunoassays (LFIAs) and Nucleic acid LFA (NALFA).4 To increase detection sensitivity, novel reagents, such as nano-gold microspheres or immune-nanoparticles, are often used.
Thermal contrast, laser, or light-emitting diode (LED) are also used to amplify signal strength.4 LFA technology has enabled the development of easy-to-use self-test kits that can cost-effectively detect multiple medical conditions at home.
Developmental regulations and availability of at-home test kits
Many manufacturers worldwide, including Abbott, ARUP Laboratories, Resolution Bioscience Inc., Leica Biosystems, Myriad Genetic Laboratories, Roche Molecular Systems Inc., and Foundation Medicine Inc., offer at-home test kits. At present, a growing list of at-home testing kits is commercially available to the general public to determine food allergies, ovulation, diabetes, respiratory viruses, sexually transmitted diseases (e.g., chlamydia, gonorrhea, HIV, syphilis, and trichomoniasis), and many more.
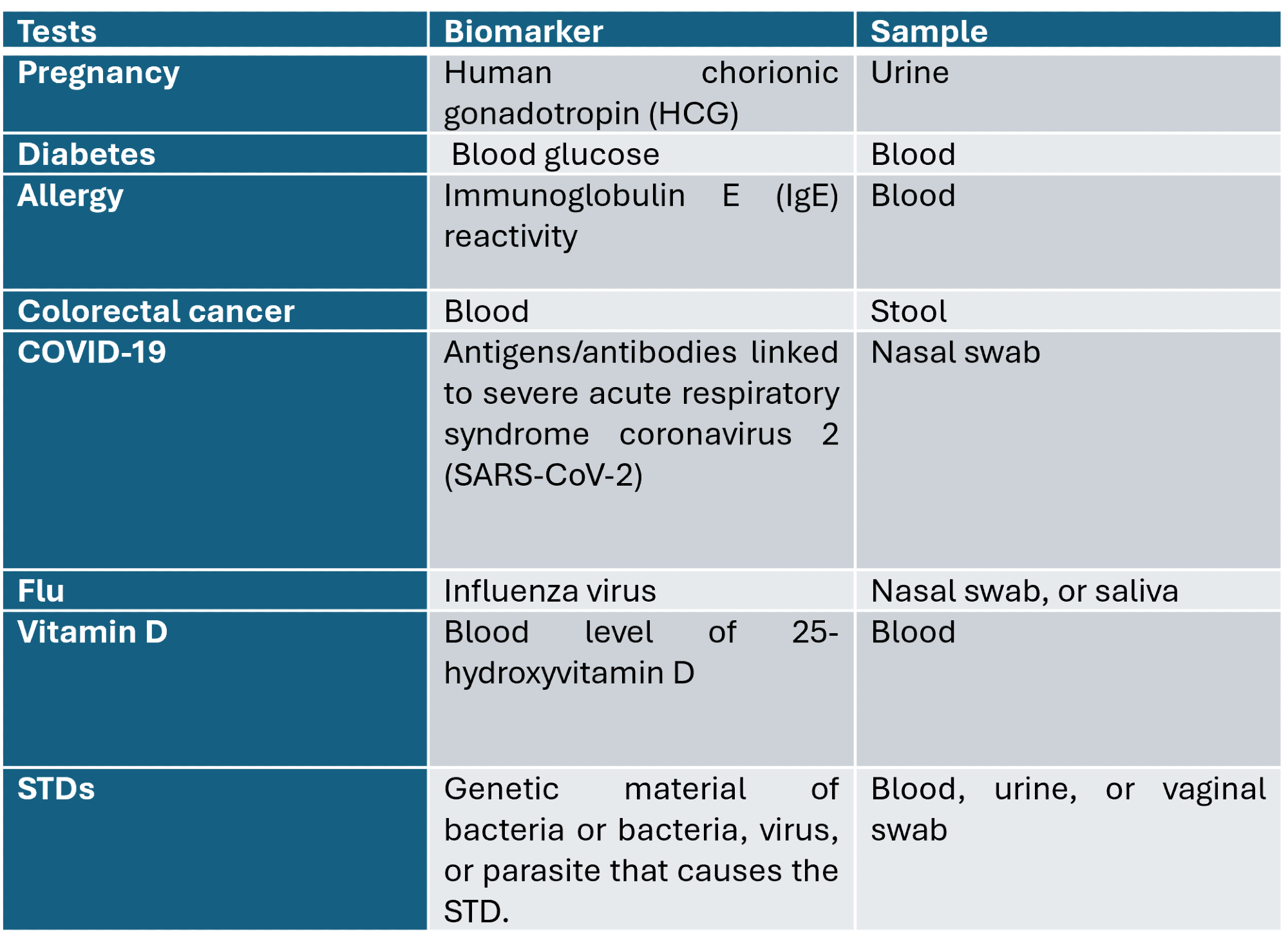
Table 1: Examples of at-home test kits and diagnostic features
Manufacturers are required to follow strict regulations and international standards while developing at-home test kits. Although the majority of self-test kits are classified as low-risk, some higher-risk self-test kits are commercially available, including HIV test kits that require extensive evaluation by the notified body.
In the UK and European Union, self-test diagnostic devices are independently reviewed by a Notified Body, such as the British Standard Institution (BSI) and Germany-based Technischer Überwachungsverein (TUV), aka Technical Inspection Association, before the device is sold commercially to the general public.5
In the UK, a new at-home testing device must be registered with the Medicines and Healthcare Products Regulatory Agency (MHRA). Self-testing kits are regulated by the In-vitro Diagnostics Directive (IVDD).6
Benefits and ends of at-home test kits
The main advantage of at-home test kits (e.g., cholesterol testing and hepatitis testing) is the possibility of being assessed even before symptoms appear. Early detection provides an opportunity for quicker intervention, which lowers the chances of complications.7
At-home test kits are in high demand because people tend to avoid embarrassing consultations, particularly for the diagnosis of STDs. Furthermore, these tests provide the convenience of disease diagnosis at home.
Reassurance is an important motivating factor that draws numerous individuals to perform routine health checks just out of curiosity. These tests also exhibited time and cost-saving benefits.
The main concern of self-testing has been the argument that disease diagnosis can rarely be made via a single test result without accounting for a patient's symptoms and clinical history. Some clinicians are concerned that false positive results of self-test kits could substantially increase healthcare providers' burden.
What measures can you follow to get reliable results?
Before using any at-home test kits, it is important to understand the possibility of false positive or negative results. False positive results trigger unnecessary worry and lead to futile investigations or doctor's appointments. In contrast, false negative results could indicate individuals to be healthy when they require immediate medical attention.
To minimize inaccurate or false positive results, you must always purchase test kits that are approved or authorized by global regulatory bodies, such as the US Food and Drug Administration. It is essential to follow the test instructions precisely, as even a minor alteration can influence the test results. Always check the expiration dates of the test kits before using them; this is important because some chemicals lose their effectiveness over time.
References
- Ibitoye M, et al. Home testing past, present and future: lessons learned and implications for HIV home tests. AIDS Behav. 2014;18(5):933-49. doi: 10.1007/s10461-013-0668-9.
- Nwofor, R. Margaret M. Crane – How One Idea Impacted Women around The World. Frontiers for Young Minds. 2022; Available at: https://www.frontiersin.org/news/2022/04/20/children-in-science-margaret-m-crane-how-one-idea-impacted-women-around-the-world
- Omidfar K, et al. Lateral Flow Assay: A Summary of Recent Progress for Improving Assay Performance. Biosensors (Basel). 2023;13(9):837. doi: 10.3390/bios13090837.
- Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem. 2016;60(1):111-20. doi: 10.1042/EBC20150012.
- Medical Devices Regulations: Routes to market and in vitro diagnostic devices. 2024; Available at: https://assets.publishing.service.gov.uk/media/672cf23c0207c4664564ce45/Medical_Devices_Consultation_November_2024.pdf
- BSI: An In Vitro Diagnostics Notified Body. A guide to the In Vitro Diagnostic Directive. Available at: https://www.bsigroup.com/globalassets/localfiles/en-hk/medical%20device/bsi-md-ivd-diagnostic-directive-guide-brochure-uk-en.pdf
- Tidy EJ, et al. Home self-testing kits: helpful or harmful? Br J Gen Pract. 2018;68(673):360-361. doi: 10.3399/bjgp18X698021.
Further Reading
Last Updated: Jan 15, 2025