This article is based on a poster originally authored by L. Fäs, F. Wenz, M. Tu, K. Sanchez, N. Zapiórkowska-Blumer, H. Varga and K. Kaczmarska, which was presented at ELRIG Drug Discovery 2024 in affiliation with InSphero AG.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Background and Purpose
The development of therapeutic drugs is hindered by hepatotoxicity. Early detection of hepatotoxic compounds during the development process allows for better resource allocation toward viable therapeutic candidates.
However, current safety assessments often fail to accurately predict hepatotoxicity in humans, resulting in the premature discontinuation of numerous drugs at advanced developmental stages.
Micro-physiological systems are in vitro models designed to accurately replicate physiological organ features, providing better predictive power than less physiological systems. Human liver microtissues, which are spherical co-cultures of primary parenchymal and non-parenchymal liver cells, effectively recapitulate key liver characteristics and predict hepatotoxicity more accurately than traditional planar hepatocyte cultures.
Methods
To further evaluate the relevance of the hepatic spheroids for hepatotoxicity assessment, the cytotoxicity (cellular ATP IC50) of 152 US Food and Drug Administration (FDA)-approved small molecule drugs in human liver microtissues was compared with their hepatotoxicity and total peak plasma concentration.
Results
The drugs' cytotoxicity correlated well with all three types of classification reported in the DILIrank dataset, an FDA resource: DILIconcern class, liver injury description, and hepatotoxicity-related warning.
Notably, 86 % of withdrawn drugs and 78 % of those associated with fatal hepatotoxicity were accurately identified as hepatotoxic, while 85.3 % of innocuous drugs were correctly classified as non-hepatotoxic.
Conclusions
The correlation between the in vitro cytotoxicity and the hepatotoxicity of the tested drugs shows the utility of human liver microtissues for detecting hepatotoxic compounds early in the drug development process.
Materials, methods & references
In vitro cell-based model
- 3D InSightTM Human Liver microtissue1
- Ten-donor pool primary human hepatocyte
- Crude NPC fraction
- Chemically defined culture media
- AKURATM 384 spheroid microplate
Cellular ATP IC50 assay
- 7-day culture and drug treatment
- Three drug applications on days 0, 2 and 5
- Cellular ATP measurement on day 7
- CellTiter-GloTM 3D Cell Viability Assay (Promega)
Data analysis
In vitro Cellular ATP IC50 data values were normalized by the human total peak plasma concentration [Cmax], and the resulting ratio [C2C] was compared to the hepatotoxicity-related warning, drug label liver injury description, and FDA’s DILIrank2 class.
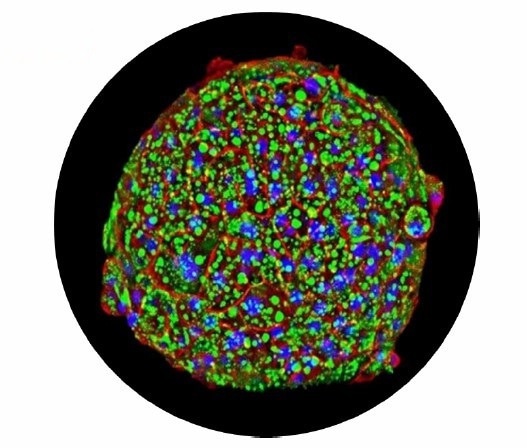
High-content microscopic image of the 3D InSight Human Liver microtissue. Image Credit: Image courtesy of L. Fäs et al., in partnership with ELRIG (UK) Ltd.
Results – Part 1
Test set: 152 FDA-approved orally administered drugs
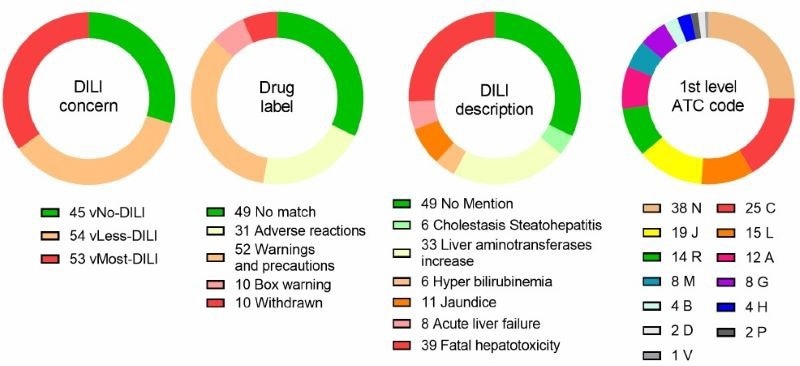
Figure 1. Description of the 152 FDA-approved drug set. Schematic representations of the drugs in relation to the DILIconcern class, hepatotoxicity-related warning (Drug label), DILI description on the drug label (DILI description), and anatomical/pharmacological groups (1st level ATC code). A: Alimentary tract and metabolism, B: Blood and blood forming organs, C: Cardiovascular system, D: Dermatologicals, G: Genito-urinary system and sex hormones, H: Systemic hormonal preparations, excluding sex hormones and insulins, J: Antiinfectives for systemic use, L: Antineoplastic and immunomodulating agents, M: Musculo skeletal system, N: Nervous system, P: Antiparasitic products, insecticides and repellents, R: Respiratory system, S: Sensory organs, V: Various. Image Credit: Image courtesy of L. Fäs et al., in partnership with ELRIG (UK) Ltd.
Cytotoxicity measurements and cytotoxicity-to-Cmax [C2C] calculations
The in vitro Cellular ATP IC50 values of hepatotoxic and safe drugs are not significantly different (Fig.2, left panel). HHowever, most hepatotoxic drugs exhibit a distinct profile when considering in vivo exposure (Fig. 2, central panel).
In agreement, the in vitro ATP IC50 to total peak plasma concentration ratios [C2C] of vMost-DILI-concern drugs and safer drugs are different (Fig.2, right panel, p-value <0.0001).
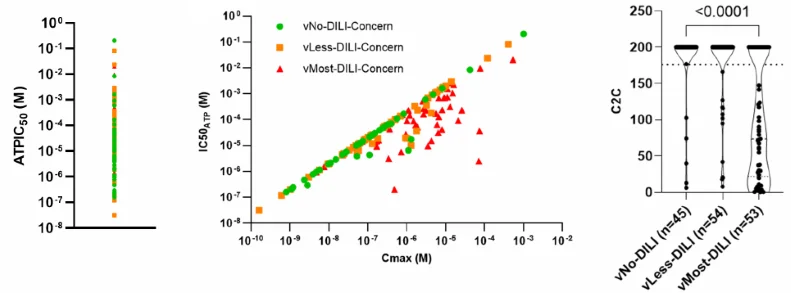
Figure 2. Cytotoxicity in human liver microtissues. Left panel: ATP IC50 values of the test set classified according to their DILIconcern class ( : vNo-DILI-concern,
: vNo-DILI-concern,  : vLess-DILI-concern,
: vLess-DILI-concern,  : vMost-DILI-concern). Middle panel: The IC50 ATP values are plotted against the human total plasma Cmax of FDA-approved drugs. Right panel: The cytotoxicity to total plasma Cmax ratio (C2C) of 152 FDA-approved drugs are plotted against their DILIconcern class (Dunn's multiple comparisons tests, p-value). Image Credit: Image courtesy of L. Fäs et al., in partnership with ELRIG (UK) Ltd.
: vMost-DILI-concern). Middle panel: The IC50 ATP values are plotted against the human total plasma Cmax of FDA-approved drugs. Right panel: The cytotoxicity to total plasma Cmax ratio (C2C) of 152 FDA-approved drugs are plotted against their DILIconcern class (Dunn's multiple comparisons tests, p-value). Image Credit: Image courtesy of L. Fäs et al., in partnership with ELRIG (UK) Ltd.
Result – Part 2
C2C score in relation to hepatotoxicity-related warning
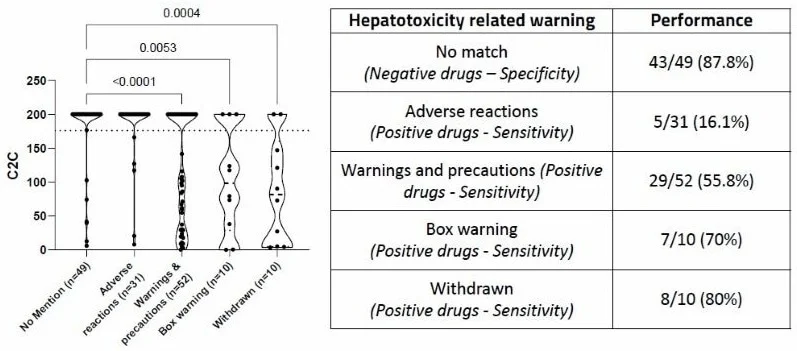
Figure 3. C2C score in relationship with drug label information. Left panel: C2C score of 152 FDA-approved drugs plotted against their hepatotoxicity safety warning (Dunn's multiple comparisons tests, p-values). Dotted line: 176. Right panel: Performance of the 176 C2C threshold to classify drugs as positive (<176) or negative (>176). Negative drugs: number of true negative/number of drugs (% of true negative). Positive drugs: number of true positive/number of drugs (% of true positive). Image Credit: Image courtesy of L. Fäs et al., in partnership with ELRIG (UK) Ltd.
Summary and conclusions
152 FDA-approved orally administrated drugs with well-documented liver liabilities were used to assess the relevance of cytotoxicity measurements in spherical cultures of primary liver cells as a measure of hepatotoxicity.
The cytotoxicity-to-total peak plasma concentration ratio (C2C) varies between hepatotoxic and safe drugs, with a C2C threshold of 176 effectively distinguishing hepatotoxic compounds.
- “Most-DILI-concern” drugs: 71.7 % sensitivity; 88.9 % specificity
- “Withdrawn” drugs: 80 % sensitivity; 87.8 % specificity
- “Box warning” drugs: 70 % sensitivity; 87.8 % specificity
- •“Warnings and precautions” drugs: 55.8 %; 87.8 % specificity
The cytotoxicity of a drug in 3D InSightTM liver microtissues in combination with a total peak plasma concentration correlates well with clinical hepatotoxicity.
This study employs a simple, spherical cell-based model that is fast (7-day), cost-effective (cytotoxicity measurement), scalable (384-well plate format), and robust (seven concentrations with four biological replicates each). This approach enables early rank-ordering of drug candidates for hepatotoxicity and assesses the risk associated with specific total peak plasma drug concentrations.
The specificity and sensitivity of 3D InSightTM liver microtissues make them valuable tools for liver safety assessment, and their cost-effectiveness, reproducibility, and throughput are compatible with industrial drug development process requirements.
References
- Messner, S., Agarkova, I., Moritz, W. and Kelm, J.M. (2012). Multi-cell type human liver microtissues for hepatotoxicity testing. Archives of Toxicology, 87(1), pp.209–213. https://doi.org/10.1007/s00204-012-0968-2.
- Chen, M., et al. (2016). DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discovery Today, 21(4), pp.648–653. https://doi.org/10.1016/j.drudis.2016.02.015.
About InSphero
InSphero is the only 3D in vitro model company with the platform and expertise to modernize drug discovery in a way that empowers researchers to reach their full potential.
Their innovative spheroid-based models allow for more effective, safe therapies to reach the clinic through truly superior robustness, reliability, reproducibility, and scalability.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 18, 2024