This article is based on a poster originally authored by Carl Haslam.
Design, Make, Test, and Analyze (DMTA) screening involves generating compound assay-ready plates (ARP). However, this process can be time-consuming, resource-intensive, and expensive, often adversely affecting project cycle times and stretching available resources.
Leveraging a powerful combination of automation from SPT Labtech and dedicated software, the team at Artios have developed in-house compound management (CM) for DMTA processing and plate generation. This tool offers several benefits:
- A new, fully automated workflow for biochemical and cellular assays
- A trackable and audited process, improving sample and data integrity
- A streamlined approach, reducing cycle times and associated costs
- Faster data reporting, enabling smooth drive decision-making and more effective resource use
Advantages of in-house CM plate supply
External plate supply and logistics
The graphs below show times from legacy sample registration to test date. This generally comprised of assay-ready plates from the CRO.


Image Credit: SPT Labtech
Cost of assay-ready plates
The image below highlights the legacy external and internal costs for plate generation.

Image Credit: SPT Labtech
The example presented here offered several benefits to Artios:
- Cycle times and costs were reduced due to direct supply to Artios.
- Sample control and tracking capabilities improved due to the employed sample management system.
- Streamlined generation of assay plates for DMTA, Kinetic, and Mechanistic formats.
- The use of automated workflows and analysis integration reduces human intervention and associated human error.
Artios’ in-house compound management workflow
Compounds are initially prepared as DMSO solutions before being imported into the Mosaic Sample Bank system and compound collection.
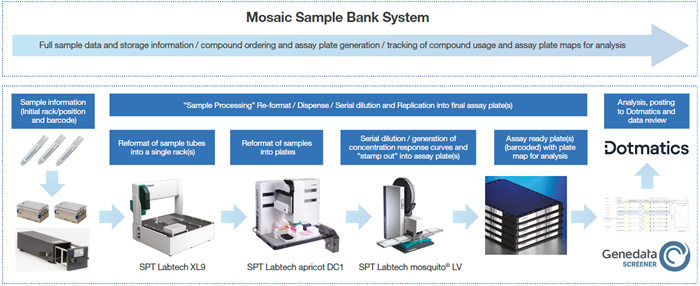
Image Credit: SPT Labtech
CM plate generation quality
It was observed that both biochemical and cellular assays demonstrated comparable compound activity and assay quality.

Image Credit: SPT Labtech
DMTA cycle times
When using the more streamlined system, up to a 40 % reduction in median cycle time (registration to data posting) was observed. The figures below show timings from the in-house CM workflow based on typical biochemical assays.
- Registration to shipping at CRO: Typically 1 day
- Shipping from CRO to Artios: Typically 7-10 days
- Assay-ready plate generation to data posting: Typically 1-2 days

Image Credit: SPT Labtech
Compound registration to Data Posting. Source: SPT Labtech
| |
|
| Legacy assay ready plates supply process (Days)* |
21 |
| In-House CM assay ready plate supply process (Days)* |
13 |

Image Credit: SPT Labtech
Summary and future applications
Artios was able to develop and embed an end-to-end workflow designed for sample storage and tracking, requesting, processing, plate generation, and sample maps for analysis. Using this workflow, the team benefited from the ability to pick samples and generate assay-ready plates for 96- and 384-well assay formats, including DMTA, Kinetic, and Mechanistic formats.
The capabilities resulted in more rapid plate generation and screening, faster data reporting to drive decision-making, and more effective financial and FTE resource use. Artios commented:
“The compound management system has definitely been a big positive and aligns us with industry standard.”
“The CMG has definitely had a positive impact……We are now receiving compounds from the CRO and typically getting results from the FRET assay by the end of the following day (sometimes same day!).”
The Mosaic tool and related automation systems can also be used for CMC sample tracking, protein crystallography, and high-throughput reagent generation.
Acknowledgments
Produced from materials originally authored by Carl Haslam from Artios Pharma.
About SPT Labtech

We Design and Manufacture Robust, Reliable and Easy-to-Use Solutions for Life Science
We enable life scientists through collaboration, deep application knowledge, and leading engineering to accelerate research and make a difference together. We offer a portfolio of products within sample management, liquid handling, and multiplexed detection that minimize assay volumes, reduce material handling costs and put the discovery tools back in the hands of the scientist.
At the heart of what we do
Many of our innovations have been born out of the desire to create solutions to existing customer problems; and it’s this ethos that drives SPT Labtech’s R&D efforts. Our strengths come from the trust our customers have with us to develop truly unique, automated technologies to meet their needs. We combine cutting edge science with first-rate engineering to put customers at the heart of everything we do.
A problem-solving state of mind
The substantial breadth of expertise within our company enables us to be involved in the full life cycle of our products from the initial design concept, mechanical and software engineering and prototyping, to final manufacture and sale. These qualities allow us to offer the best possible technical and mechanical support to all the equipment that we supply, hence maintaining excellent client relationships.
SPT Labtech Company Overview
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.