Reviewed by HH Patel, M.Pharm.
By Nita Sharma Das, PhD, ND
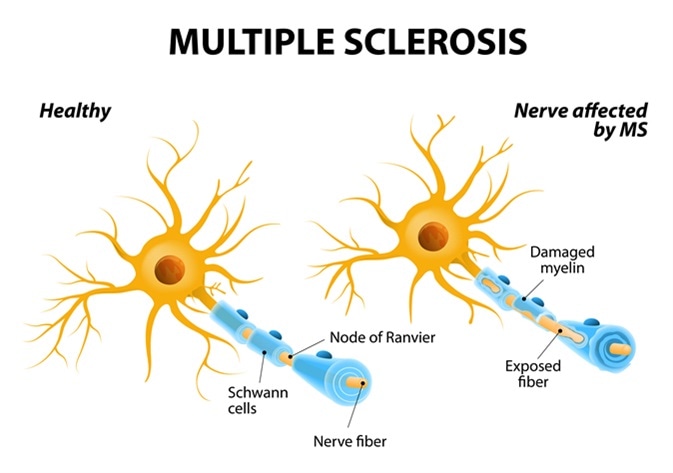
Multiple sclerosis (MS) is an auto-immune disease which affects the myelin sheath surrounding the axon, causing axonal degeneration and neuronal loss.
Details of the pathogenesis of this disease have not been fully established, but it is known to cause demyelination in the central nervous system, causing neuronal loss and gliosis. It is primarily caused by autoreactive T lymphocytes, which target myelin and myelin oligodendrocyte glycoprotein.
Multiple sclerosis or MS. autoimmune disease. the nerves of the brain and spinal cord are damaged by one's own immune system resulting in loss of muscle control, vision and balance. Image Credit: Designua / Shutterstock
According to the literature, microglial activation can also cause chronic neurodegeneration. This phenomenon is called gliosis.
There are other environmental risk factors which can cause MS. Epigenetic alterations due to genetic predisposition are also responsible for MS.
Epigenetic Involvement in the Development of MS
DNA methylation is an important biomarker for identification of MS, as it affects the activity of lymphocytes. DNA methyltransferases (DNMTs) are enzymes which are commonly present in brain tissues, and are required for DNA methylation. DNMTs attach additional methyl groups to cytosine bases in DNA.
DNA methylation changes gene expression as it modifies the structure of chromatin, restricting access by transcriptional enzymes.
Dysregulation of specific micro RNAs (miRNAs) is responsible for neuromodulation and modulation of immune processes resulting in the onset of MS. Following are some common epigenetic involvements that can be responsible for MS development:
- Epigenetic changes cause DNA methylation and lead to MS progression. 20% to 30% monozygotic twins suffer from MS, whereas 5% individuals with alike-sex dizygotic twins suffer from MS.
- In the genomic structure, Chromosome 6 becomes susceptible to MS. According to research publications, the human leukocyte antigen (HLA)-DRB1*15:01 locus on Chromosome 6 plays an important role in MS development.
- Genetically predisposed individuals lack prominent blood-brain barrier, which results in the development of plaque in the white and gray matter. Plaque or lesion is formed due to loss of myelin within CNS of the brain, and is one of the primary attributes of MS.
- Each genetic material in human locus contains two alleles, each of which is inherited from either of the parents. Therefore, a genetically inherited trait of an allele of an MS-affected individual depends on the gene inherited from the mother or the father. This causes a prominent involvement of a major histocompatibility complex (MHC) in the MS-affected patient. Allele HLA-DRB1*15 is considered as a major risk factor for MS, which can be preferentially transmitted from mothers to affected female offsprings.
- Epigenetic involvement influences exacerbation or reduction of symptoms associated with MS.
- When any definite genetic involvement is not identified through genetic mapping, it is termed as “missing heritability”, which can be explained by the role of epigenetic mechanisms in MS development.
Illustration of Epigenetic Involvement of MS Through Different Research Findings
There are several investigational studies conducted to identify epigenetic involvement of MS. Enzyme peptidyl arginine deiminase 2 promoter has hypomethylated CpG islands in the brain, which increases the level of citrullinated myelin basic protein, and further promotes histone acetylation. Citrullinated myelin basic protein is very unstable and prone to faster degradation than the normal myelin basic protein.
Normal appearing white matter (NAWM) present in brain tissues of MS patients contains an increased level of citrullinated myelin basic protein. Increased level of acetylation of histone H3 in NAWM results in discontinuing re-myelination of nerves in patients with chronic MS.
Additionally, increased acetylation of histone H3 is responsible for enhancing the expression of transcriptional inhibitors of oligodendrocyte differentiation. 5-hydroxymethylcytosine (5hmC) acts as a DNA methylation promoter, and a significant reduction of 5hmC in peripheral mononuclear cells causes MS.
While assessing the methylation status of DNA, it has been found that 220 and 319 genetic regions in NAWM get extensively hypo-methylated and hyper-methylated, respectively.
Dysregulation of miRNAs leads to a pro-inflammatory condition in MS disease progression. The miR-155 and miR-326 are two identified specific miRNAs which are up-regulated in MS.
Further Reading
Last Updated: Aug 23, 2018