This article is based on a poster originally authored by Zhen Qi, Yingying Fu, Spencer Chiang, Wei Li , Huihui Han, Zhanguang Zuo, Shuge Guan, Mary Spanou, Idil Arioz, Cheng Wang and Rosanna Zhang.
Current organoid technologies tend to lack a range of specific physiological cell types, as well as translatable biological assays and standardized protocols.
Despite these limitations, organoids offer excellent potential in drug development and disease modeling due to their potential to address the high clinical drug failure rates that remain commonplace, even after rigorous preclinical testing.1
Organoids are widely regarded as the gold standard preclinical drug development model due to their translatable human genomic background, potential for high throughput, and ability to mimic their respective organs' function and three-dimensional architecture.2
Using organoids as preclinical models can notably improve drug development pipeline efficiency while reducing reliance on animal models that regularly fail to predict human responses with sufficient accuracy.
ACROBiosystems has developed novel, commercially available, hydrogel-free human iPSC-derived assay-ready cerebral and cardiac organoid systems.
Cerebral and cardiac organoids each bear a range of cell types verifiable via marker immunolabeling and RNA-seq and exhibit the functional characteristics of the organs they model (verifiable via patch clamp, MEA, and silicon probe recording).
Using these cerebral organoids, a Parkinson’s disease model was created by adding alpha-synuclein pre-formed fibrils. The cardiac organoid was also used as a model to test a drug’s cardiac toxicity.
These organoids represent extremely promising models for AAV screening and gene therapy development. ACROBiosystems’ advances in the field are leading to the provision of better guidance for disease and toxicity modeling and gene therapy development as these progress into clinical research.
Methods and materials
iPSC and organoid generation
Commercially purchased human iPSCs (Cat. No. ACS-1007, ATCC) were used to create organoids. This was done in line with the protocol outlined in the product’s datasheet, using ACROBiosystems’ cerebral and cardiac organoid kits (Cat. No. RIPO-BWM001K, RIPO-HWM002K, respectively).
Harvested iPSCs were initially seeded onto a 96-well ultra-low attachment plate, and EB formation was monitored. The media included in the kit was used to treat the cells to prompt organoid formation and cell differentiation.
Organoids were employed in assays according to the specified time points. They were fixed, permeabilized, and immunolabeled with the indicated cell markers to enable cell marker confirmation.
In the Parkinson’s disease model, cerebral organoids were treated with 0.1 µM and 1 µM alpha-synuclein pre-formed fibrils (Cat. No. ALN-H5115) for 12 days, commencing on day 92.
In the cardiac toxicity model, cardiac organoids were treated with different concentrations of drug A (drug name redacted) on day 25.
In the case of the AAV transduction, cerebral organoids and cardiac organoids were transduced with 1011 vg of eGFP transgene-bearing AAV5 WT as a negative control or with optimized AAV serotypes containing IVB-1 and IVB-2. This was done on 101 days and 11 days, respectively.
Organoids were incubated at 37 °C at 5% CO2 in every instance, continually shaking these at 100 rpm until they were ready to be analyzed.
Results
Characterization of cerebral organoids: Cell marker verification
ACROBiosystems’ Cerebral Organoid Differentiation kit was used to produce human iPSC-derived cerebral organoids (Figure 1A) before characterization.
Characterization involved immunolabeling for (Figure 1B to Figure 1E):
- Neural progenitor and immature neuronal cells at day 36
- Dopaminergic neurons and mature neurons at day 92
- Astrocytes at day 109
- Oligodendrocytes at day 119
- Microglia at day 147
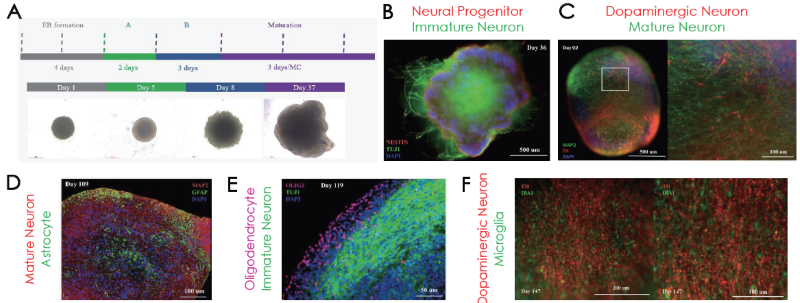
Figure 1. (A) Cerebral organoid differentiation steps. Immunofluorescence confirmation of (B) NESTIN and TUJ1, (C) MAP2 and TH, (D) GFAP, (E) OLIG2 and (F) IBA1 positive cells on different stages of organoid maturation. Image Credit: ACROBiosystems
Characterization of cerebral organoids: Transcriptomic and functional characterization
Cerebral organoids exhibit several neuronal subtypes and glial cell types, which can be verified using bulk RNA-seq (Figure 2A). They also demonstrate extracellular electrical activity, which can be verified via MEA analysis (Figure 2B) and silicon probe recording (Figure 2C).
It is also important to note that silicon probe recording revealed a range of neuronal groups in early network activity in the example presented here (Figure 1D), while patch clamp was used to verify intracellular electrical activity.
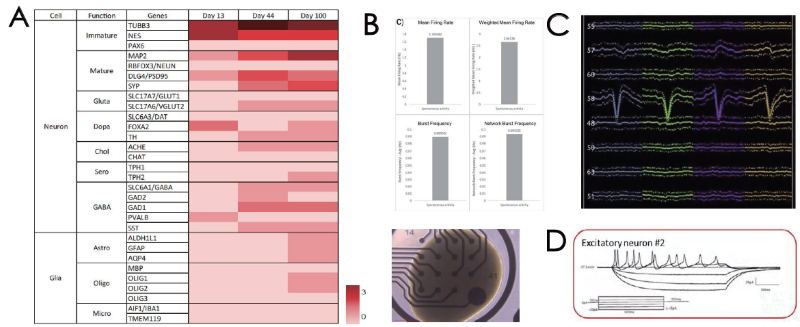
Figure 2. (A) Transcriptome profiling of cerebral organoids. Extracellular electrical activity by (B) MEA and (C) silicon probe recording. (D) Intracellular electrical activity by patch clamp. Image Credit: ACROBiosystems
Parkinson’s disease and AAV capsid screening modeling on cerebral organoids
The treatment of healthy 92-day-old cerebral organoids with alpha-synuclein pre-formed fibrils for a total of 12 days triggered the degeneration of dopaminergic neurons and disruption of other neurons' dendrites (Figure 3A, left). This occurred in a concentration-dependent fashion in each case (Figure 3A, right).
In another instance, cerebral organoids were grown for 101 days before being infected with AAV5-WT, IVB-1, and IVB-2. The IVB-2 subtype demonstrated higher infectiousness and transgene delivery to cerebral organoids.
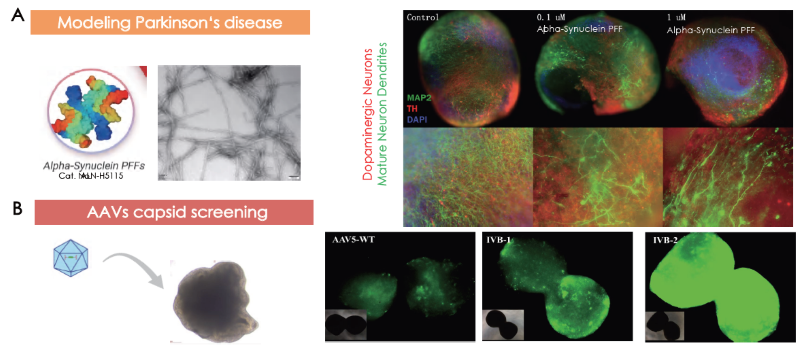
Figure 3. Cerebral organoids as models for (A) Parkinson’s disease and (B) AAV capsid screening. Image Credit: ACROBiosystems
Characterization of cardiac organoids: Cell marker verification
ACROBiosystems’ Cardiac Organoid Differentiation kit (Figure 4A) was used to produce human iPSC-derived cardiac organoids. These were then characterized by immunolabeling for cardiomyocytes on day 12 (Figure 4B) and mechanical beating (Figure 4C, beating not shown here).
Mechanical beating became visible following 9 days of culturing in cardiac organoids. Calcium sensor imaging confirmed that calcium flux behavior is present in human heart organoids (Figure 4D).
Cardiac organoids also present ventricular and atrial-like chambers in different regions. These morphological features were validated using ventricular and atrial cardiomyocyte markers (Figure 4E). The presence of endothelial cells was also confirmed on the cardiac organoids (Figure 4F).
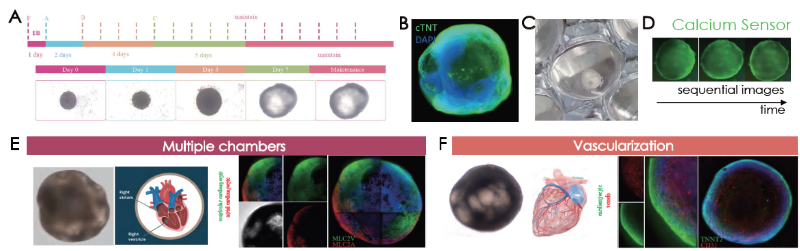
Figure 4. (A) Cardiac organoid differentiation steps. Immunofluorescence confirmation of (B) cTNT and (B) visualization of cardiac organoids on tissue culture plates. (D) Sequential images of calcium sensor imaging of cardiac organoids. (E) Presence of morphological and marker validated atrial (MLC2A+ cells) and ventricular (MLC2V+ cells) chambers. (E) Vascularization was confirmed by CD31 positive endothelial cell immunolabeling. Image Credit: ACROBiosystems
Characterization of cardiac organoids: Functional characterization
The heart tissue was observed to be electrically active, with MEA recordings employed to verify the presence of electrical activity. This probe evaluated consistent beating periods and amplitudes of the cardiac organoid model before these were compared against three other cardiac models over 6-hour time periods (Figure 5A).
It is possible to measure extracellular electrical activity using silicon probe recordings. This confirmed the presence of physiological wave complex elements for heart activity (Figure 5B).
Patch clamp was also used to verify intracellular electrical activity, allowing a physiological cardiac waveform to be identified (Figure 5C).
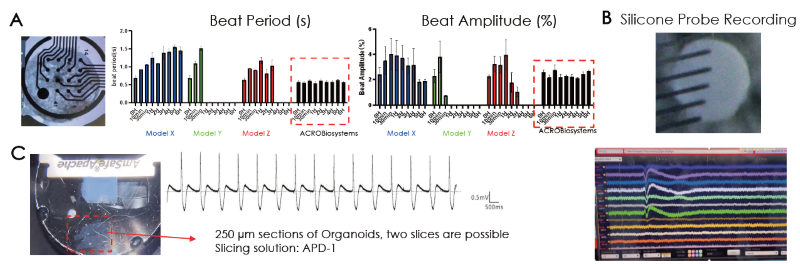
Figure 5. (A) Electrical activity of the cardiac organoids were confirmed extracellularly by (A) MEA and (B) silicon probe recordings and intracellularly by (C) patch clamp recordings. The resemblance of a physio logical waveform of cardiac beating was identified. Image Credit: ACROBiosystems
Cardiac toxicity and AAV capsid screening modeling on cardiac organoids
Healthy 25-day-old cardiac organoids were treated with an appropriate concentration of drug A (name redacted). This prompted the degeneration of cardiomyocytes and endothelial cells (Figure 6A).
In a further study, cardiac organoids grown for 11 days were infected with AAV5-WT, IVB-1, and IVB-2. The IVB-2 subtype displayed higher rates of infectiousness and transgene delivery toward cerebral organoids, as visualized using fluorescent intensity from GFP transgene expression.
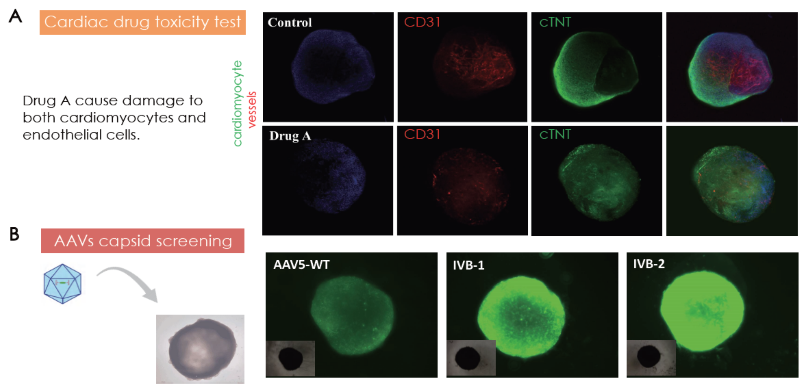
Figure 6. (A) Cardiac toxicity model by Drug A treatment on cardiac organoids. (B) Cardiac organoids as models for AAV capsid screening. Image Credit: ACROBiosystems
Conclusion
Pharmaceutical drug development is prone to high clinical failure rates due to side effects and efficacy issues, even following stringent preclinical testing. These issues can be attributed to insufficient relevant and translatable cell models.
Organoids are useful three-dimensional in vitro tissue models that mimic their respective organs’ architecture and function. As such, organoids represent a promising new drug development and disease modeling tool, offering significant potential in combatting traditionally high clinical drug failure rates.
Current organoid technologies generally lack certain physiological cell types, as well as translatable biological assays.
ACROBiosystems can offer commercially available human iPSC-derived cerebral and cardiac organoids that are suitable for use as models for diseases, toxicity, and AAV screening.
Cerebral organoids bear several neuronal subtypes, with these and all glial cell types displaying functional activity. Cardiac organoids have also been shown to bear all relevant cell types, as well as being functionally active and demonstrating mechanical beating.
It was possible to create a Parkinson’s disease model by adding alpha synuclein pre-formed fibril to cerebral organoids, recapitulating the dopaminergic neuronal degeneration phenotype. It was also noted that cardiac organoids degenerated following the addition of a cardiotoxic drug, whereby it was possible to observe the cardiomyocyte and endothelial degeneration phenotype.
Both organoids were found to be promising models for AAV screening for gene therapy developments. ACROBiosystems asserts that its cerebral and cardiac organoids represent excellent preclinical models for gene therapy, drug screening, drug toxicity, and disease modeling.
References and further reading
- Kim, J., Koo, B.-K. and Knoblich, J.A. (2020). Human organoids: model systems for human biology and medicine. Nature Reviews Molecular Cell Biology, (online) 21(10), pp.571–584. https://doi.org/10.1038/s41580-020-0259-3.
- Zhao, Z. (2022). Organoids. Nature Reviews Methods Primers, (online) 2(1), pp.1–21. https://doi.org/10.1038/s43586-022-00174-y.
Acknowledgments
Produced from materials originally authored by Yingying Fu, Wei Li, Huihui Han, and Cheng Wang from Innovec Biotherapeutics; and Zhen Qi, Spencer Chiang, Zhanguang Zuo, Shuge Guan, Mary Spanou, Idil Arioz, and Rosanna Zhang from ACROBiosystems.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Feb 18, 2025