This article is based on a poster originally authored by Sibusiso T. Malindisa, Tshireletso Mentor, and Monde M. Ntwasa, which was presented at ELRIG Drug Discovery 2024 in affiliation with University of South Africa (UNISA).
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Ezetimibe, primarily used as a cholesterol absorption inhibitor,1 is being repurposed as an orally administered cancer drug. In silico studies have shown that ezetimibe, in its parent form, binds to the p53 binding domain of MDM2, potentially inhibiting the ubiquitination and degradation of p53.2
Ezetimibe is metabolized via phase II glucuronide conjugation in small intestines and liver to form its main phenolic metabolite, ezetimibe-glucuronide.1,3 Curcumin, known for its anticancer properties,4 can inhibit the metabolism of ezetimibe, enhancing its bioavailability in its parent form, which is crucial for its anticancer effect.
This study highlights the anticancer potential of ezetimibe and its synergistic effects with curcumin, specifically in colorectal cancer models. The combination therapy has been named EzecurminTM.

Image Credit: Sibusiso T. Malindisa et al., in partnership with ELRIG (UK) Ltd.
Methodology
- In silico Pharmacokinetics: Pharmacokinetic prediction using Schrodinger’s Qikprop
- In vitro real-time cytotoxicity: Real-time cell analysis assay (XCELLIgence)
- In vivo Preclinical study: Female NMRI nude mice implanted with HCT116 colon carcinoma
Result and discussion
In silico pharmacokinetics
Ezetimibe exhibits high oral absorption, but curcumin and ezetimibe-glucuronide exhibit a moderate and low degree of oral absorption.
Table 1. QikProp pharmacokinetic prediction of ezetimibe, curcumin, and ezetimibe-glucuronide in comparison to Nutlin-3a and RG7112. Pharmacokinetic prediction using Schrodinger’s Qikprop. Properties include Molecular weight, number of HB donor and acceptors, number of likely metabolic reactions, number of reactive functional groups, octanol/water partition coefficient, aqueous solubility, IC50 value for blockage of HERG K + channels, apparent Caco-2 cell permeability in nm/sec, brain/blood partition coefficient, apparent MDCK cell permeability in nm/sec, prediction of binding to human serum albumin, percent human oral absorption, number of violations of Lipinski’s rule of five, number of violations of Jorgensen’s rule of three.
| |
NUTLIN-3a |
RG7112 |
EZETIMIBE |
EZETIMIBE-
GLUCURONIDE |
CURCUMIN |
| mol MW |
581.497 |
699.734 |
409.432 |
585.557 |
368.385 |
| donorHB |
1 |
0 |
2 |
4 |
2 |
| accptHB |
7.5 |
10.25 |
4.45 |
12.25 |
5.5 |
| #metab |
5 |
4 |
3 |
6 |
1 |
| #rtvFG |
0 |
0 |
1 |
2 |
8 |
| QPlogPo/w |
4.648 |
6.003 |
5.137 |
3.252 |
1.278 |
| QPlogS |
-5.592 |
-6.563 |
-6.856 |
-6.359 |
-3.574 |
| QPlogHERG |
-2.126 |
-5.222 |
-6.836 |
-5.407 |
-4.265 |
| QPPCaco |
370.536 |
158.995 |
612.453 |
3.61 |
529.874 |
| QPlogBB |
-0.244 |
-0.282 |
-0.909 |
-3.349 |
-0.851 |
| QPPMDCK |
2829.648 |
614.173 |
948.183 |
4.693 |
249.008 |
| QPlogKHSA |
0.572 |
0.964 |
0.857 |
-0.081 |
-0.201 |
Percent
Human Oral
Absorption |
87.178 |
75.58 |
93.946 |
43.006 |
70.227 |
| RuleOfFive |
1 |
2 |
1 |
1 |
1 |
| RuleOfThree |
0 |
1 |
1 |
2 |
0 |
The results show that Ezetimibe exhibits greater oral absorption and Caco-2 cell permeability than Nutlin-3a or RG7112. Curcumin, however, only exhibits greater Caco-2 cell permeability compared to Nutlin-3a and RG7112. Once ezetimibe is metabolized, its pharmacokinetic profile is not advantageous.
In vitro real-time cytotoxicity
Ezetimibe and Ezecurmin were seen to induce cytotoxicity in colorectal cancer cells (HCT116) with wild-type p53.
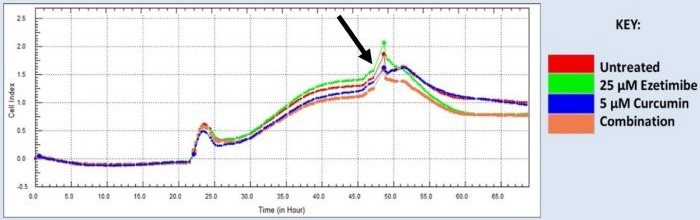
Figure 1. Real-time cell proliferation analysis of HCT116 cells treated with ezetimibe and curcumin. Real-time cell proliferation analysis of HCT116 cells treated with ezetimibe and curcumin. Arrow indicated addition of drug treatments. Image Credit: Sibusiso T. Malindisa et al., in partnership with ELRIG (UK) Ltd.
A sharp decline in the cell index was observed in HCT116 cells treated with ezetimibe and Ezecurmin, which is a total contrast to the continuous growth observed in all other treatment groups for at least three hours.
Ezecurmin was found to slow down proliferation in colorectal cancer cells with mutant p53 (HT-29 and SW620).
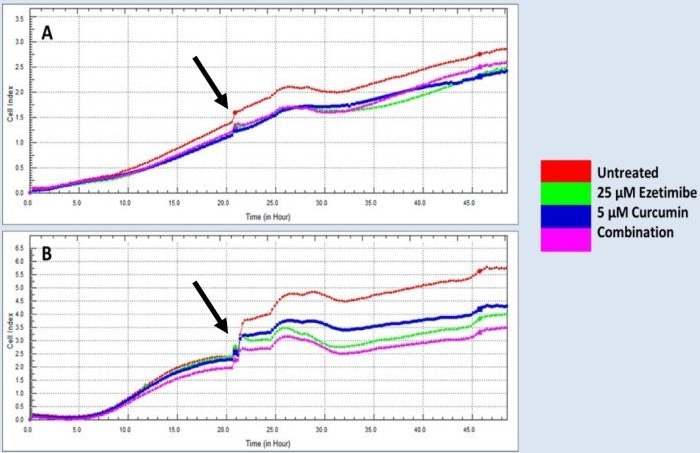
Figure 2. Real-time cell proliferation analysis of human colorectal cancer cells lines treated with ezetimibe and curcumin. A) HT-29 cell line with the cell index not affected by the single and combination treatments. B) SW620 cell line with the cell index not affected by the single and combination treatments. Arrow indicated addition of drug treatments. Image Credit: Sibusiso T. Malindisa et al., in partnership with ELRIG (UK) Ltd.
Ezecurmin and ezetimibe alone were able to slow down the growth of SW620 cancer cells. However, none of the treatments significantly impacted the growth of HT-29 cells. Notably, both HT-29 and SW620 cells carried the R273H Tp53 mutation, with an additional P309S mutation present in SW620.
In vivo preclinical study
Ezetimibe alone was found to induce weight loss in female NMRI nude mice implanted orthotopically with human HCT-116_Luc colon carcinoma tumor.
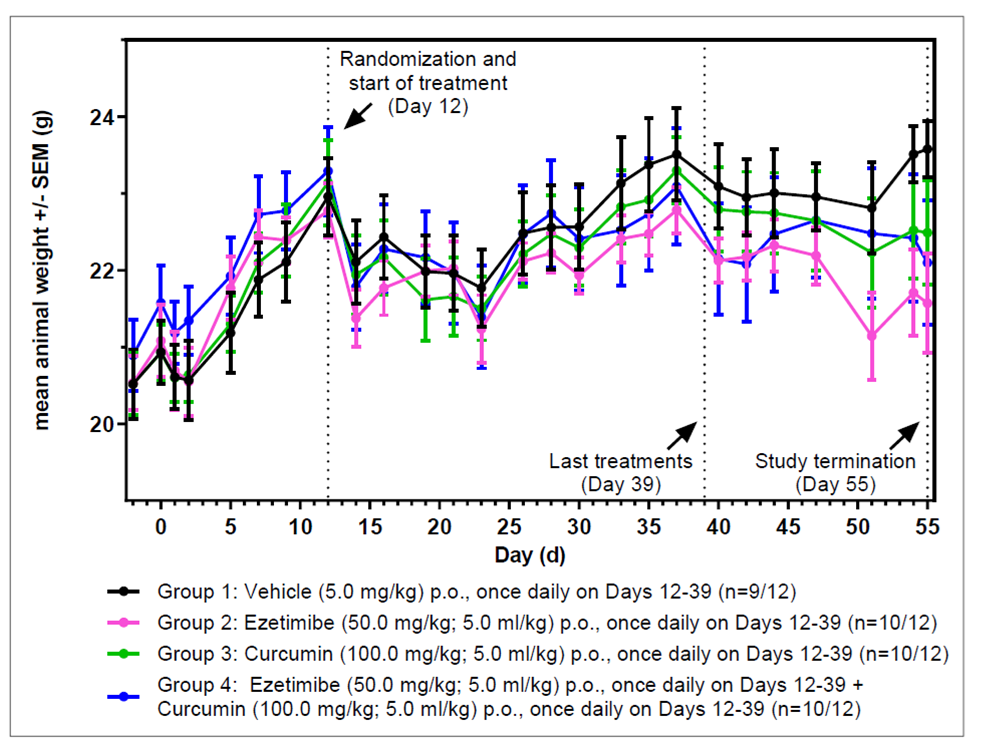
Figure 3. Animal weight over time (g): On Day 0, female NMRI nude mice were implanted orthotopically (caecum) with human HCT-116_Luc colon carcinoma tumor cells and were treated as depicted in the figure. Data is displayed as means +/- SEM. Image Credit: Sibusiso T. Malindisa et al., in partnership with ELRIG (UK) Ltd.
Ezecurmin also reduced tumor size in mice from day 35 post-treatment.
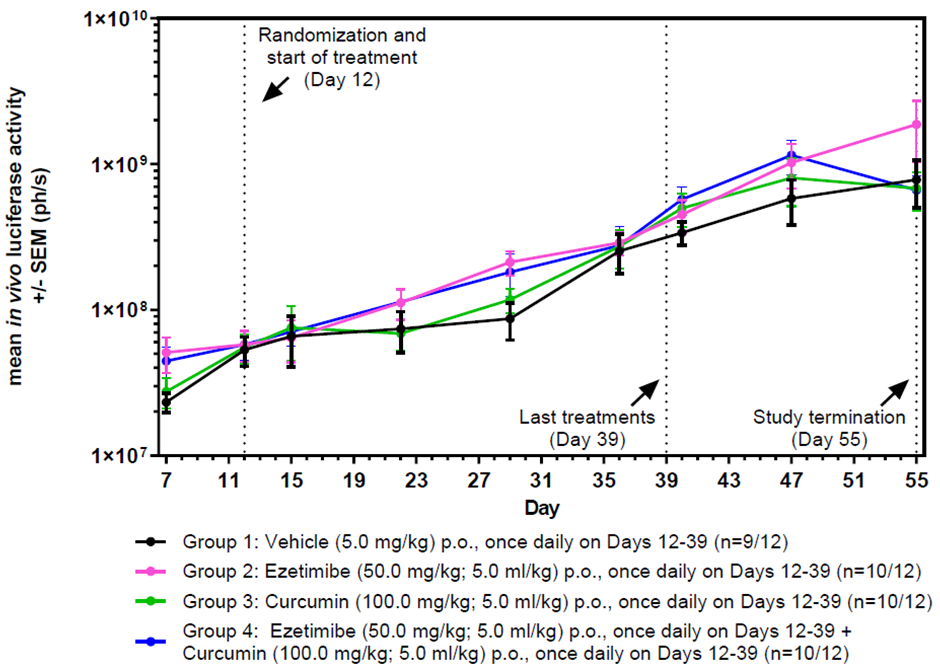
Figure 4. In vivo luciferase activities in Female NMRI nude mice. All NMRI nude mice implanted orthotopically (caecum) with human HCT-116_Luc colon carcinoma tumor cells showing an increase in in vivo luciferase activity over 55 days. Treatment started on day 12 days post-implantation and terminated on day 39 (27 days treatment). Image Credit: ibusiso T. Malindisa et al., in partnership with ELRIG (UK) Ltd.
Conclusions
- Synergistic anticancer effect: Ezecurmin shows potent cytotoxicity in colorectal cancer cells with wild-type p53.
- p53-dependent efficacy: Efficacy is selective, with mutant p53 cell lines showing resistance to Ezecurmin.
- Tumor reduction in vivo: Ezecurmin reduces tumor size in mice from day 35 post-treatment.
- Repurposed for cancer: Ezetimibe, enhanced by curcumin, can be repurposed as an effective anticancer therapy.
- Clinical potential: Ezecurmin demonstrates promising potential for colorectal cancer treatment, warranting further clinical exploration.
Literature
- Nutescu, E.A. and Shapiro, N.L. (2003). Ezetimibe: A Selective Cholesterol Absorption Inhibitor. Pharmacotherapy, 23(11), pp.1463–1474. https://doi.org/10.1592/phco.23.14.1463.31942.
- Twala, Charmy Starnod (2017). Drugs targeting the retinoblastoma binding protein 6 (RBBP6): ‘the collision of computers and biochemistry’. [online] Wits.ac.za. Available at: https://wiredspace.wits.ac.za/items/29108526-609e-44bb-ae04-25a31c8c9360 [Accessed 24 Oct. 2024].
- Kosoglou, T., et al. (2005). Ezetimibe. Clinical Pharmacokinetics, 44(5), pp.467–494. https://doi.org/10.2165/00003088-200544050-00002.
- Tomeh, M.A., Hadianamrei, R. and Zhao, X. (2019). A Review of Curcumin and Its Derivatives as Anticancer Agents. International Journal of Molecular Sciences, [online] 20(5). https://doi.org/10.3390/ijms20051033.
Acknowledgments
The University of South Africa (UNISA) for conference funding; the National Research Foundation (NRF) and the Technology Innovation Agency (TIA) of South Africa for project funding; and the ELRIG organising committee for accepting our poster presentation.
About University of South Africa (Unisa)
The University of South Africa (Unisa), the only higher education institution to carry the name of the country, is the people's university in every sense of the word.
Throughout its history, spanning 15 decades, Unisa has responded to the developments brought about by changing times, the needs of a developing country and society at large, and an ever-evolving higher education environment.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 18, 2024