This article is based on a poster originally authored by Polina V. Rusina, Maria J. Falaguera, Juan Maria R. Romero, Ellen M. McDonagh, Ian Dunham* & David Ochoa in affiliation with the European Molecular Biology Laboratory, European Bioinformatics Institute (EMBL-EBI) Open Targets, *Wellcome Sanger Institute, Wellcome Genome Campus. This poster was presented at ELRIG Drug Discovery 2023.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Since genetic evidence can identify the underlying causes of diseases, it has frequently been used as a surrogate for drug development success. An extensive retrospective analysis of the genetic basis for drug approvals during the previous 10 years was carried out by acCELLerate.
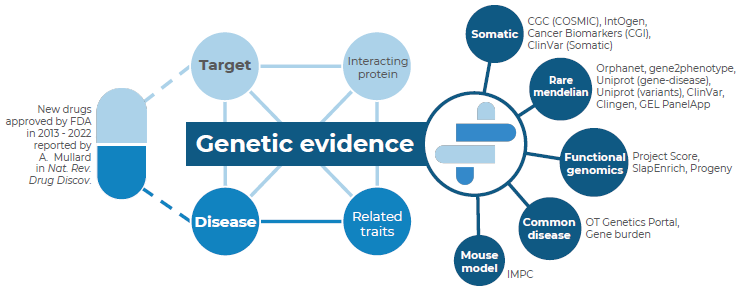
Association score > 0
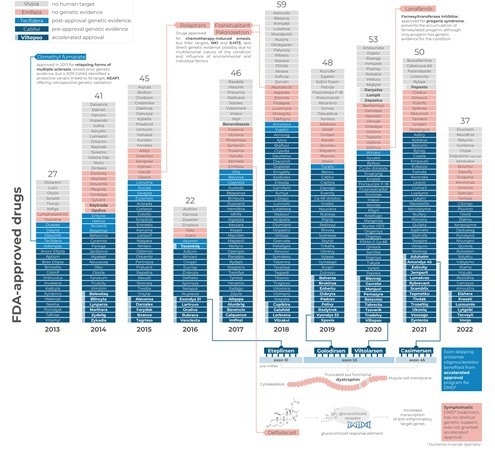
FDA-approved drugs
Genetics for acceleration?
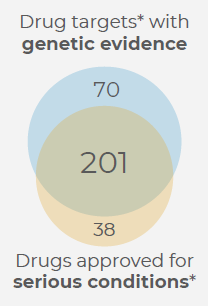
FDA-accelerated approvals were more likely to be based on genetic evidence [OR = 9.5 (2.3 - 40), p < 0.002). This correlation could indicate a focus on genetics for critical reasons, or a better knowledge of the causal relationship for faster therapies.
Last Updated: Nov 18, 2024