Ion channels are transmembrane proteins specifically involved with the transport of inorganic ions like Na+, K+, Ca2+, or Cl-. Ion channels are “gated”, i.e. they open in response to a specific stimulus, such as a change in membrane potential (voltage-gated ion channels) or the binding of a neurotransmitter (ligand-gated ion channels).
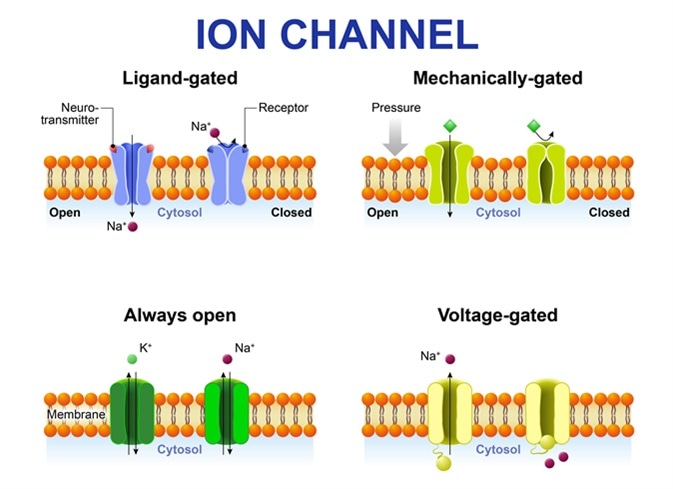
Types of ion channel. Classification by gating. mechanism of action. Voltage-Gated, Ligand-gated, Mechanically-gated and Always open ion channels. Image Credit: Designua / Shutterstock
The voltage-gated ion channels allow permeation of only one type of ion while the ligand-gated channels are less selective and allow permeation of two or more types of ions through the channel pore.
The flux of ions leads to the generation of an electrical potential difference, termed membrane potential, between the inner and outer environment of the cell. This membrane potential plays a pivotal role in a number of physiological processes in our body, such as signal transduction, muscle contraction, release of neurotransmitters, growth, motility, hormone secretion, volume regulation, and apoptosis.
Ion Channels: How your body communicates
Generation of Action Potentials in Excitable Cells (Nerve and Muscle Cells)
Voltage-gated Na+ and K+ channels are responsible for the generation of action potentials in neurons and skeletal muscle cells. In the neuron, generation of an action potential produces the nerve impulse, whereas in the muscle cell it leads to the contraction of skeletal muscle. The opening of these channels leads to the influx of positive ions into the cell, which causes depolarization. Depolarization of the membrane drives the membrane potential initially towards the Na+ equilibrium potential and then back towards the K+ equilibrium potential.
Voltage-gated Ca2+ channels play a pivotal role in the various signal transduction processes. These channels allow the entry of Ca2+ into the cytoplasm, which acts as a second messenger and initiates a number of cellular events. They are mainly involved in the contraction of cardiac and smooth muscle cells, secretion of hormones, activation of protein kinases, initiation of synaptic transmission and regulation of gene expression.
Ligand-Gated Ion Channels
Ligand-gated ion channels are a group of intrinsic transmembrane proteins that are opened by the binding of a neurotransmitter. The ligands binds to a site, which is distinct from the ion conduction pore, termed an orthosteric site. The binding causes structural modifications, which change the permeability of the ion channel, and thus allow only specific ions to pass through.
Glutamate and acetylcholine are the major excitatory neurotransmitters of the brain. Upon binding to their respective receptors, they open the transmitter-gated cation channels and cause depolarization of the postsynaptic membrane. This ultimately leads to the initiation of an action potential.
On the other hand, GABA (γ-Aminobutyric acid) and glycine are major inhibitory neurotransmitters. They are involved in the opening of transmitter-gated Cl-or K+ channels, which leads to hyperpolarization of the neuronal membrane, and causes elevation of the "threshold" required to depolarize the membrane. They keep the postsynaptic membrane polarized and suppress the generation of an action potential.
Ligand-gated ion channels are important, because they are believed to be targets for a number of drugs used to treat diseases, such such as depression, anxiety, epilepsy, Alzheimer's disease, Schizophrenia, and Autism.
Channelopathies – Disorders Due to Defects in Ion Channel Functions
Channelopathy is the term used to describe the various disorders that occur due to defects in ion channel function. These include conditions resulting from mutations in ion channels, and also disorders caused by autoimmune attacks on ion channels.
Channelopathies due to mutations in the genes encoding ion channels are more common. Such mutations impair the channel functions and result in a variety of diseases.
Channelopathies include diseases of the nervous system, the cardiovascular system (e.g., long QT syndrome, short QT syndrome), the respiratory system (e.g., cystic fibrosis), the endocrine system (e.g., neonatal diabetes mellitus, the urinary system (e.g. nephrogenic diabetes insipidus, autosomal-dominant polycystic kidney disease), and the immune system (e.g., myasthenia gravis).
Classifying channelopathies is difficult as a large amount of heterogeneity and phenotypic variations are observed among the various diseases. With the recent advances in deciphering the role of ion channels in the human body, the list of channelopathies is expanding. The treatment approach for the majority of channelopathies focuses on palliative therapy. Treatment strategies involve either treating the symptoms of the disease or usage of drugs targeting the mutated ion channel. This approach lacks efficacy. Moreover, it has tolerability issues, and usually results in unwanted or unacceptable side-effects. Gene therapy and gene editing tools can be tailored to the needs of each patient, and thus offer a new ray of hope for the treatment of channelopathies.
Further Reading
Last Updated: Oct 25, 2018