This article is based on a poster originally authored by Nermeen Ali, Mina Adly, Azza Taher, Heather Coleman, Fakher Ahmed, Ashraf Mahmoud, Mohamed Salem and Rana El-Masry, which was presented at ELRIG Drug Discovery 2024 in affiliation with Ulster University, Cairo University, October 6 university and University for Modern Sciences and Arts.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Introduction
Fluoroquinolones are a class of compounds with known antimicrobial properties, but their potential as anticancer agents remains underexplored.1,2 DNA topoisomerase has been identified as important molecular targets for anticancer drugs.3
Objective
This study aims to design and synthesize novel fluoroquinolone analogs derived from Ciprofloxacin hydrazide, with the goal of discovering potent and selective anticancer agents. These compounds are specifically designed to inhibit the Topoisomerase II enzyme, a critical player in DNA replication and cell cycle regulation, thereby serving as a promising target for anticancer therapies.
Method
A series of fluoroquinolone analogs were synthesized and subjected to the NCI-60 Human Tumour Cell Line Screening assay to assess their cytotoxicity. The most potent compounds were further tested in a five-dose assay. Topoisomerase II inhibition assays were conducted to elucidate the mechanism of action. Selectivity was assessed by comparing the cytotoxic effects on cancerous NCI-60 cell lines versus normal mammalian Vero cells. Cell cycle analysis was performed on MCF-7 cells to investigate the effects on cell cycle distribution.
Results
The novel derivatives demonstrated a remarkable reduction in cell proliferation across most tested cell lines, with IC50 values significantly lower than Etoposide, exhibiting 4-12 folds greater cytotoxicity.
Derivative I, in particular, displayed high selectivity for cancer cells, with markedly reduced toxicity to Vero cells (CC50 = 349.03 µM) compared to its potent cytotoxic effect on cancer cells (Mean GI50 = 9.06 µM). Topoisomerase II inhibition assays confirmed that Ciprofloxacin hydrazide derivatives are potent inhibitors of the enzyme. Furthermore, cell cycle analysis revealed that the most active Ciprofloxacin hydrazide derivatives induced cell cycle arrest at the G2/M phase in MCF-7 cells.
Graphs
Image Credit: Image courtesy of Nermeen Ali, in partnership with ELRIG (UK) Ltd.
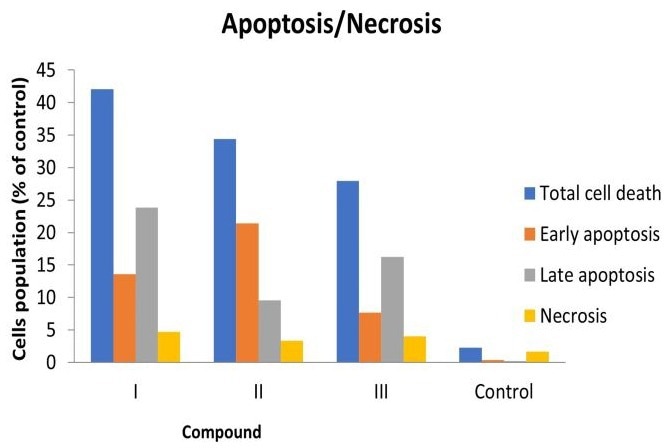
Image Credit: Image courtesy of Nermeen Ali, in partnership with ELRIG (UK) Ltd.
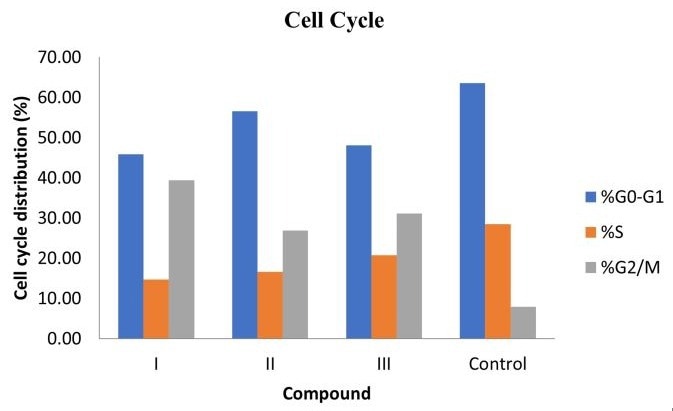
Image Credit: Image courtesy of Nermeen Ali, in partnership with ELRIG (UK) Ltd.
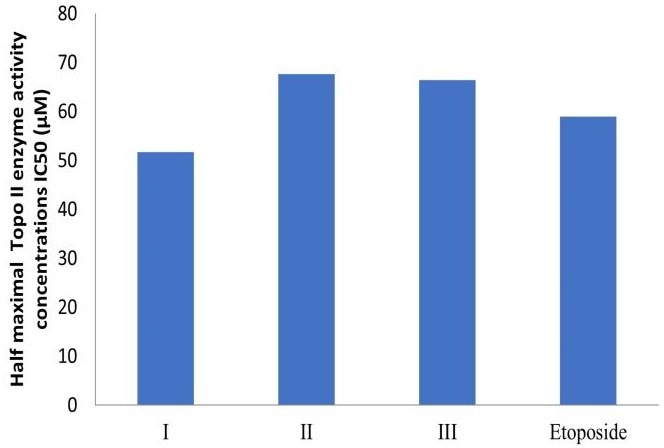
Image Credit: Image courtesy of Nermeen Ali, in partnership with ELRIG (UK) Ltd.
Cell study results. Source: Ulster University
| |
Ciprofloxacin |
Ciprofloxacin
hydrazide
derivatives I |
Ciprofloxacin
hydrazide
derivatives
II, III |
Etoposide |
Mean GI50 of
NCI-60 cell lines
(µM) |
3.30 |
9.06 |
2.45 |
2.85 |
CC50 of Vero cell
line (µM) |
30.62 |
349.03 |
43.45 |
|
Full NCI panel SI
(using mean GI50) |
9.28 |
38.52 |
17.73 |
Conclusion
A new series of fluoroquinolone-based compounds were designed and synthesized as potential anticancer agents. A single-dose NCI 60 cell line screen revealed that most of these novel derivatives exhibited significant potency across the tested lines.
The five-dose assay demonstrated broad-spectrum cytotoxicity comparable to Etoposide, with several compounds showing remarkable reductions in cell proliferation across most tested cell lines.
These derivatives achieved IC50 values significantly lower than Etoposide, resulting in up to 4- to 12-fold greater cytotoxicity. In the normal VERO cell line cytotoxicity assay, most compounds were found to be selectively more toxic to cancer cell lines than to normal cells.
Cell cycle analysis further revealed that the most active compounds disrupted the cell cycle phase distribution in MCF-7 cells, inducing arrest in the G2/M phase. Additionally, a Topoisomerase II inhibition assay confirmed that these target molecules exhibit potent inhibitory activity against the Topoisomerase II enzyme, supporting their proposed mechanism of action.
In conclusion, these findings suggest that these compounds are potential candidates for the development of new anti-cancer agents. Future work will focus on designing additional analogs to establish a more advanced structure-activity relationship and optimize their therapeutic potential.
References
- Sung, H., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a Cancer Journal for Clinicians, [online] 71(3), pp.209–249. https://doi.org/10.3322/caac.21660..
- Bray, F., et al. (2024). Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A cancer journal for clinicians, 74(3), pp.229–263. https://doi.org/10.3322/caac.21834.
- Buzun, K., et al. (2020). DNA topoisomerases as molecular targets for anticancer drugs. Journal of Enzyme Inhibition and Medicinal Chemistry, 35(1), pp.1781–1799. https://doi.org/10.1080/14756366.2020.1821676.
About Ulster University
Ulster University has a national and international reputation for excellence, innovation and regional engagement and continues to make a major contribution to the economic, social and cultural development of Northern Ireland.
Ulster has four campuses across the Northern Ireland - Belfast, Coleraine, Jordanstown and Magee. The university believes in making higher education accessible for all students.
Ulster’s core business activities are teaching and learning, widening access to education, research and innovation and technology and knowledge transfer.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 20, 2024