This article and associated images are based on a poster originally authored by Zhong Yu, Parker Ellingson, Denise Sullivan, Ben Streeter, Austin Passaro, Stacie Chvatal and Daniel Millard and presented at ELRIG Drug Discovery 2024 in affiliation with Axion BioSystems.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Multiwell MEA technology
Microelectrode array technology
The complexity and difficulty of measuring signals from the brain make the in vivo study of neurological disorders and toxicological drug screening laborious and costly. In vitro neural cultures provide an alternative to in vivo studies and allow for high throughput analysis of neurological disease states and neurotoxic and seizurogenic assessment of drugs. Measurements of electrophysiological activity across a networked population of cells provide a comprehensive view of function beyond standard characterization through genomic and biochemical profiling.
Axion BioSystems’ MaestroTM multiwell microelectrode array (MEA) platform offers such a solution by providing a label-free, non-invasive bench-top system to simply, rapidly, and accurately record functional activity from a population of cells cultured on an array of extracellular electrodes in each well.
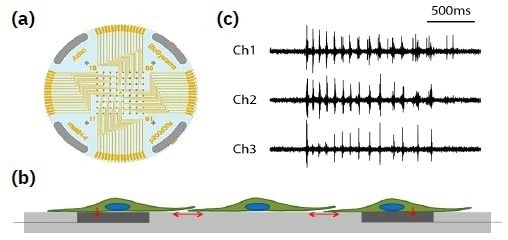
A planar grid of microelectrodes (a) interfaces with cultured neurons (b), modeling complex, human systems over an electrode array. Electrodes detect changes in raw voltage (c) through recording of extracellular field potential. Image Credit: Image courtesy of Zhong Yu et al., in partnership with ELRIG (UK) Ltd.
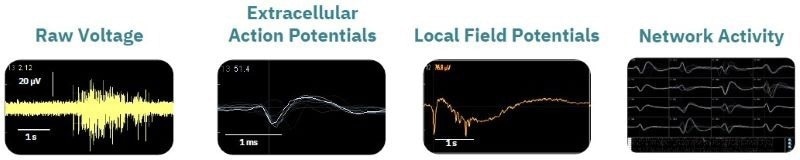
Image Credit: Image courtesy of Zhong Yu et al., in partnership with ELRIG (UK) Ltd.
Raw voltage signals are processed in real time to obtain extracellular field potentials across the network, providing a valuable electrophysiological phenotype for applications in drug discovery, toxicological and safety screening, disease models, and stem cell characterization.
The Maestro MEA product family
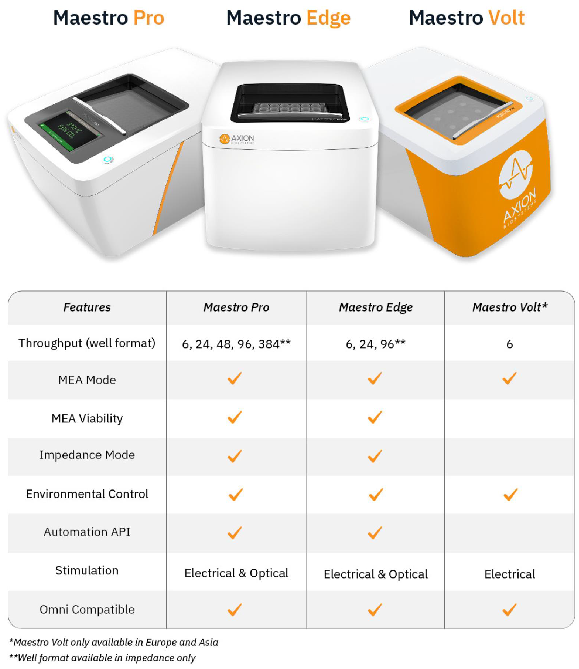
Image Credit: Image courtesy of Zhong Yu et al., in partnership with ELRIG (UK) Ltd.
- Label-free, non-invasive tracking extracellular voltage from cultured electro-active cells.
- Integrated environmental control provides a stable benchtop environment for short- and long-term toxicity studies
- Fast data collection rate (12.5 kHz) accurately quantifies the depolarization waveform
- Sensitive voltage resolution detects subtle extracellular action potential events
- Industry-leading array density provides high-quality data from across the entire culture
- Scalable format (6-, 24-, 48- and 96-well plates) meets all throughput needs on a single system
- State-of-the-art electrode processing chip (BioCore v4) offers stronger signals, ultra-low frequency content, and enhanced flexibility
MEA assay with neurons
Structure and function in one assay
The Maestro comprehensively assesses neuronal activity, network connectivity, and structural integrity.
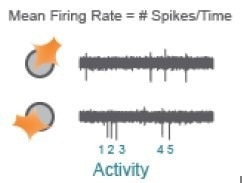
Image Credit: Image courtesy of Zhong Yu et al., in partnership with ELRIG (UK) Ltd.
Action potentials are the defining feature of neuron function. Mean Firing Rate captures the frequency of action potentials produced by neurons.
Image Credit: Image courtesy of Zhong Yu et al., in partnership with ELRIG (UK) Ltd.
Synchrony reflects the influence of functional synapses between neurons, indicating the likelihood of one neuron firing an action potential in response to another neuron.
Image Credit: Image courtesy of Zhong Yu et al., in partnership with ELRIG (UK) Ltd.
Functional networks with excitatory and inhibitory neurons exhibit network-level oscillations, alternating between high and low levels of network-wide activity.
Image Credit: Image courtesy of Zhong Yu et al., in partnership with ELRIG (UK) Ltd.
Cell coverage and viability can be monitored using impedance-based technology on the same microelectrodes used to measure neuronal function.
Development of in vitro neural activity over time
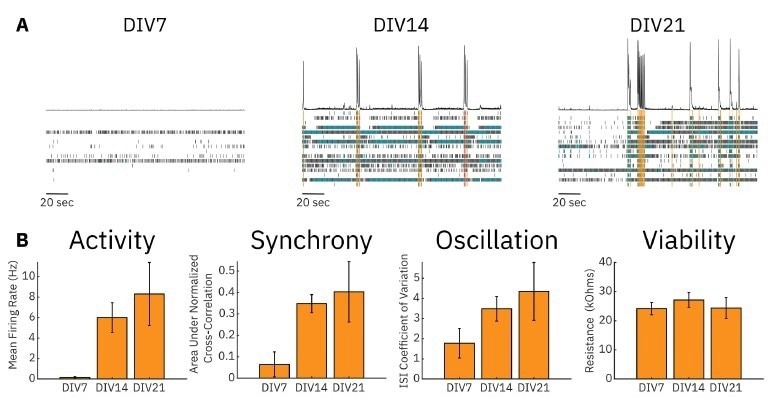
Image Credit: Image courtesy of Zhong Yu et al., in partnership with ELRIG (UK) Ltd.
To monitor rodent cortical neuron (RCN) cultures in vitro, we plated 40,000 cells per well of a 24-well CytoView MEA plate. We cultured the RCNs in BrainPhysTM Neuronal Medium with the NeuroCultTM SM1 Neuronal Supplement for 21 days.
Example raster plots of neural activity illustrate that uncoordinated, low-rate spiking occurred in RCN cultures at DIV7, while more synchronous and highly active cultures were present at DIV14 and DIV21 (A).
Metrics quantifying RCN cultures' overall activity, synchrony, and oscillations were also calculated. Mean firing rate (MFR) was low at DIV7 but increased significantly at DIV14 and further still by DIV21. The same increase over time was seen for the area under the normalized cross-correlation and the inter-spike interval (ISI) coefficient of variation, measures of culture synchrony, and oscillatory behavior, respectively. We also measured the resistance (viability) of the RCN cultures and found no significant change over 21 days (B).
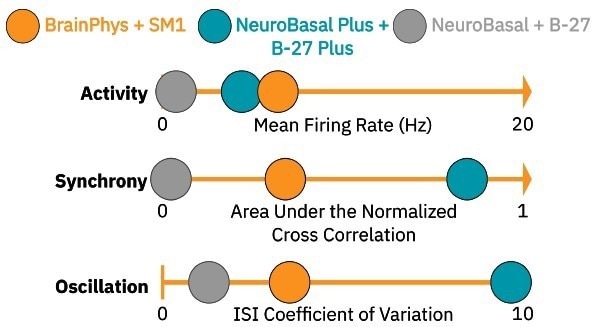
Image Credit: Image courtesy of Zhong Yu et al., in partnership with ELRIG (UK) Ltd.
Above is a pictograph displaying the three characteristics of our RCN cultures’ neural activity and their quantitative measures at DIV14 in different media types.
NeuroBasalTM Plus and B-27TM Plus media resulted in highly synchronous and oscillatory cultures (teal circles), while NeuroBasalTM + B-27TM media caused the cultures to produce significantly less activity, synchrony, and oscillations (gray circles).
We chose BrainPhysTM + NeuroCultTM SM1 in this experiment as a culture media (orange circles) because it led to a moderate level of baseline neural activity for each measure, allowing for the detection of either increases or decreases in all three parameters following compound dosing.
While our chosen cell density (40,000 cells/well), media type, and dosing time were optimal for our model and application, other cell densities, media, and time courses may be more appropriate for other cell models or applications.
Optimized neural compound dosing
Rodent cortical neuron response to neuroactive compounds
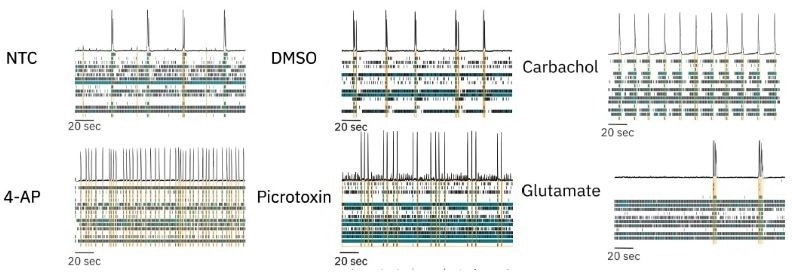
Image Credit: Image courtesy of Zhong Yu et al., in partnership with ELRIG (UK) Ltd.
We dosed RCN cultures in BrainPhys + SM1 with several neuroactive compounds with different mechanisms of action and assessed the ability of our culture model to detect changes in activity, synchrony, and oscillations.
We dosed RCN cultures with a no-treatment control (NTC, media) and vehicle control (DMSO) and found no appreciable changes in neural activity compared to baseline. To modulate neural activity, we dosed RCN cultures with four different compounds: picrotoxin, 4-aminopyridine (4-AP), carbachol, and glutamate. Raster plots collected from dosed neural cultures illustrate that each compound induced distinct, significant changes in neural activity.
Quantification of changes in neural network activity
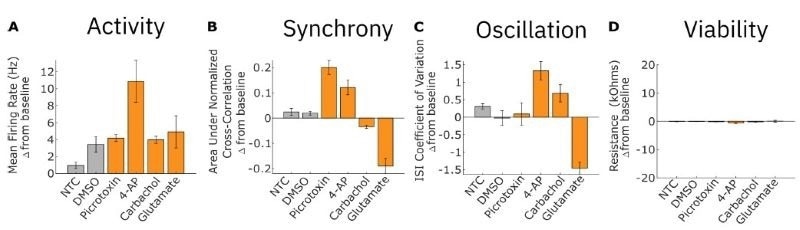
Image Credit: Image courtesy of Zhong Yu et al., in partnership with ELRIG (UK) Ltd.
We quantified activity, synchrony, and oscillation measures and plotted their changes post-dose relative to baseline values. Dosing with 4-AP, a potassium channel blocker, led to increases in activity, synchrony, and oscillation in RCN cultures.
In contrast, dosing with glutamate, an excitatory neurotransmitter, led to a large increase in activity but in a highly asynchronous manner, leading to decreases in synchrony and oscillation in the RCN culture. We also dosed RCN cultures with picrotoxin, a GABA antagonist and pro-convulsant, and carbachol, a cholinergic agonist.
Picrotoxin dosing led to increased synchrony but did not affect oscillatory behavior. In contrast, carbachol dosing led to a small decrease in synchrony but significantly increased oscillations. All dosing results for each electrophysiological metric are shown. Resistance did not significantly decrease for any treatment condition, indicating that changes in neural activity were not due to cytotoxicity from the dosed compounds (D).
About Axion BioSystem
Axion BioSystems’ suite of live-cell analysis tools offers next-generation solutions for continuous, noninvasive monitoring of in vitro cell models on your benchtop or inside your incubator. From investigating disease mechanisms in neurology and cardiology to developing lifesaving immunotherapies and antivirals, Axion’s award-winning products are designed to accelerate scientific breakthroughs and transform therapeutic development from discovery to manufacturing.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 18, 2024