Plasma donation is a specific form of blood donation whereby plasma, the clear liquid component of whole blood, is collected during donation through a process known as plasmapheresis. During plasmapheresis, an apheresis machine removes and separates the plasma from the whole blood. As plasma contains nutrients, proteins, and antibodies, it can be used to develop treatments for diseases. Plasma used for these purposes is called convalescent plasma.
 A bag of blood plasma. Image Credit: Pavel Kosolapov / Shutterstock.com
A bag of blood plasma. Image Credit: Pavel Kosolapov / Shutterstock.com
Convalescent plasma has been used in the prevention and treatment of infectious diseases for centuries. It is considered a form of ‘passive immunization’ as plasma collected from patients who have recovered from a previous infection and developed adaptive immunity, demonstrated by the presence of antigen-specific antibodies. Upon transfusion into another patient, the plasma can neutralize the disease pathogen, eventually eradicating it from blood circulation.
The COVID-19 pandemic revived interest in the use of plasma donation as a potential treatment for the disease, especially prior to the development of effective vaccines. Plasma therapies offer several advantages: large volumes can be collected from donors regularly, without impacting the donor’s hemoglobin levels as their red blood cells are reinfused during the donation process. Recruiting donors and transfusing donor plasma on a regional basis can offer the added value of providing specific, passive immunity against the local strain variations of the infectious agent.
Effectiveness of plasma therapy for other coronaviruses and similar infections
Prior to the COVID-19 pandemic, convalescent plasma has been successfully used to treat Severe Acute Respiratory Syndrome (SARS), Middle Eastern Respiratory Syndrome (MERS) and the avian influenza outbreak. In the case of SARS treatment, administration of convalescent plasma was associated with lower mortality and earlier discharge from hospital if given within 14 days of the onset of illness.
In the treatment of MERS, therapy was beneficial when the administered plasma had a higher concentration of MERS-specific antibodies. Given the clinical and virological similarities between these and COVID-19, it stands to reason that convalescent plasma was investigated as a promising treatment option for COVID-19.
Treating COVID-19 with donated plasma
One early pilot study conducted in China evaluated the impact of convalescent plasma in ten patients who had been hospitalized with severe COVID-19. The study found that clinical symptoms of COVID-19 were significantly reduced in all patients, with fever, breathlessness, cough, and chest pain especially improved. Inflammatory biomarkers such as C-reactive protein also appeared reduced after transfusion.
As in earlier studies, better results were observed in those who received plasma with higher antibody levels within 14 days of infection. Overall, the group showed a much-improved health status when compared to a recent historic control group. No serious adverse events were reported following transfusion in the plasma therapy group. The authors concluded that plasma therapy could offer a low-risk therapeutic treatment option but conceded that more rigorous, randomized controlled studies were needed.
Subsequently, several observational case studies were published and reported associations between convalescent plasma and reduced viral load, improvement in clinical symptoms and reduced mortality.
Randomized controlled trials of convalescent plasma therapy and COVID-19
What is a randomized controlled trial (RCT)?
An RCT is a type of experiment which aims to reduce bias in the interpretation of results by randomly assigning trial participants to one of two conditions: experimental or control. In the experiment condition (or arm), participants receive the intervention under study whilst the control arm receives either a conventional or placebo treatment.
The two groups will then be followed up for a predetermined time to assess differences between them, either in outcome measurements or measurements of acceptability or safety. They are considered the ‘gold standard’ for determining the effectiveness of a given intervention, as they are the most rigorous method of determining a cause-effect relationship between an intervention and outcome.
The first RCT was conducted in China and randomized 103 participants to receive either plasma therapy or standard treatment. However, the trial was discontinued early as falling rates of new infections meant that the trial could not recruit any new participants after March 2020. Analysis of the clinical outcomes showed no significant differences in time to recovery or rates of mortality between the plasma therapy and control groups. However, preliminary subgroup analysis was suggestive of a clinical benefit for those with severe COVID-19. The study's early termination may have statistically underpowered the trial, and the authors suggested that larger trials should be conducted.
The second RCT aimed to address this by recruiting over 400 patients to receive either standard care or convalescent plasma therapy. The trial was designed on the assumption that participants would not have high levels of virus-neutralizing antibodies when first recruited to the study. Upon receiving plasma therapy, the hypothesis was that antibody increases would be seen in the experimental group, leading to better clinical outcomes. However, high levels of SARS-CoV-2 antibodies were found in most participants prior to randomization. The study was discontinued after the researchers concluded that those in the experimental group would be unlikely to benefit from plasma therapy.
Subsequently, an Indian study was able to recruit approximately 500 participants who were randomly allocated to receive plasma therapy or standard care and found there was no difference between the groups in progression to severe disease or mortality.
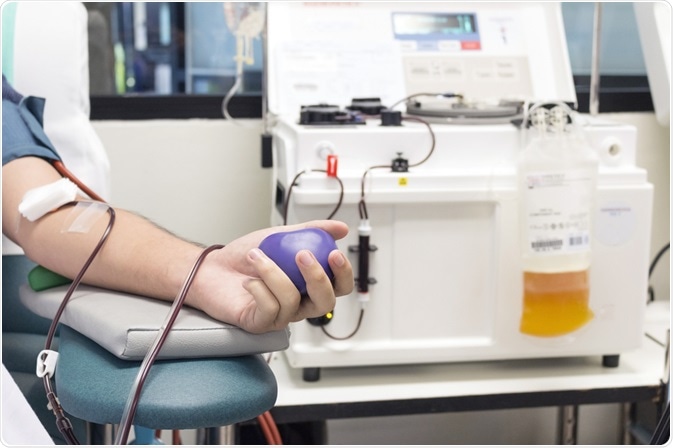 Plasma donation. Image Credit: RUCHUDA BOONPLIEN / Shutterstock.com
Plasma donation. Image Credit: RUCHUDA BOONPLIEN / Shutterstock.comIs convalescent plasma therapy ineffective in COVID-19?
Researchers have not dismissed the use of plasma therapy as a potential treatment of COVID-19, and several countries have obtained the regulatory approvals to include it as a therapy. Trials have proven methodologically difficult owing to the complexity of conducting research during the pandemic with complex patients.
Recent research as of February 2020 included plasma with COVID-19 antibody levels 6 to 10 times higher than the Indian study. Interim analysis of the data collected as part of this research indicated that receiving plasma with high antibody levels did significantly reduce mortality. Follow-up data from the same study identified that the mortality decreased significantly if delivered within an optimal 44-hour window post-hospitalization for COVID-19.
In summary, there is not yet conclusive evidence of plasma therapy’s role in COVID-19, and it has been proved to be hard to undertake trials during the pandemic for methodologic reasons.
References
- Agarwal, A., Mukherjee, A., Kumar, G., Chatterjee, P., Bhatnagar, T., Malhotra, P., & PLACID Trial Collaborators (2020). Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ (Clinical research ed.), 371, m3939. https://doi.org/10.1136/bmj.m3939
- Cheng, Y., Wong, R., Soo, Y. O., Wong, W. S., Lee, C. K., Ng, M. H., Chan, P., Wong, K. C., Leung, C. B., & Cheng, G. (2005). Use of convalescent plasma therapy in SARS patients in Hong Kong. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology, 24(1), 44–46. https://doi.org/10.1007/s10096-004-1271-9
- Duan, K., Liu, B., Li, C., Zhang, H., Yu, T., Qu, J., Zhou, M., Chen, L., Meng, S., Hu, Y., Peng, C., Yuan, M., Huang, J., Wang, Z., Yu, J., Gao, X., Wang, D., Yu, X., Li, L., Zhang, J., … Yang, X. (2020). Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proceedings of the National Academy of Sciences of the United States of America, 117(17), 9490–9496. https://doi.org/10.1073/pnas.2004168117
- Li, L., Zhang, W., Hu, Y., Tong, X., Zheng, S., Yang, J., Kong, Y., Ren, L., Wei, Q., Mei, H., Hu, C., Tao, C., Yang, R., Wang, J., Yu, Y., Guo, Y., Wu, X., Xu, Z., Zeng, L., Xiong, N., … Liu, Z. (2020). Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA, 324(5), 460–470. https://doi.org/10.1001/jama.2020.10044
- Marano, G., Vaglio, S., Pupella, S., Facco, G., Catalano, L., Liumbruno, G. M., & Grazzini, G. (2016). Convalescent plasma: new evidence for an old therapeutic tool?. Blood transfusion = Trasfusione del sangue, 14(2), 152–157. https://doi.org/10.2450/2015.0131-15
- Salazar, E., Christensen, P.A., Graviss, E.A., Nguyen, D.T., Castillo, B., Chen, J., Lopez, B.V., Eagar, T.N., Yi, X., Zhao, P., Rogers, J., Shehabeldin, A., Joseph, D., Masud, F., Leveque, C., Olsen, R.J., Bernard, D.W., Gollihar, J., Musser, J.M. (2021). Significantly decreased mortality in a large cohort of coronavirus disease 2019 (covid-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein IgG. Am J Pathol, 191(1):90-107. doi: 10.1016/j.ajpath.2020.10.008.
Further Reading
Last Updated: Mar 1, 2021