This article is based on a poster originally authored by Kris Ver Donck and Filip Delport of FOx BIOSYSTEMS and presented at ELRIG Drug Discovery 2023.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Abstract
Fiber-optic surface plasmon resonance (FO-SPR) is a powerful tool that harnesses the performance of surface plasmon resonance (SPR) in an easy-to-use dip-in fiber-optic configuration. Here, we propose an automated method for the specific detection, quantification and isolation of extracellular vesicles (EV), a particularly challenging task due to the heterogeneity of EVs and the complexity of the matrices in which they are typically found, such as culture medium or blood plasma.
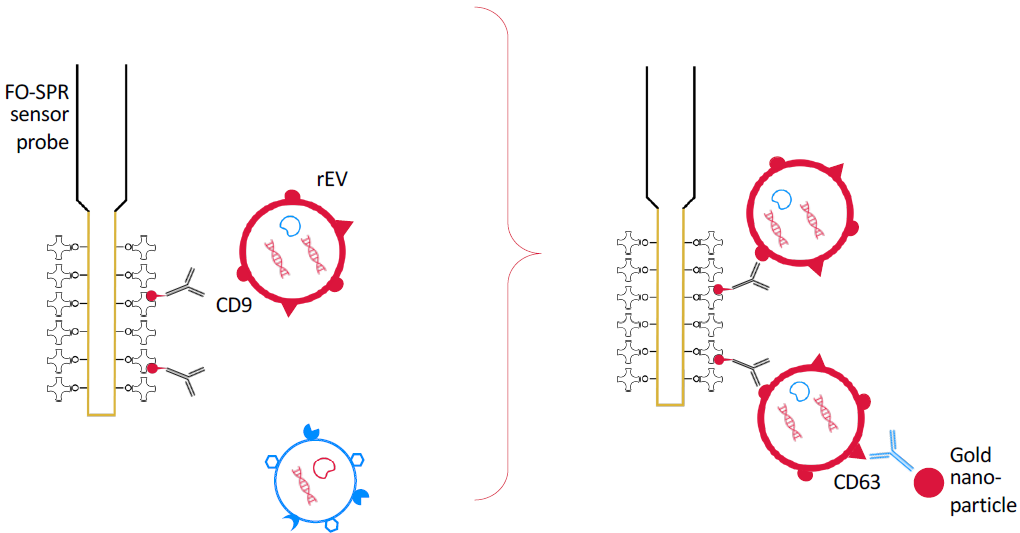
Figure 1. Real time detection. Fiber optic surface plasmon resonance (FO-SPR) dip-in probes in 96 well plates on WHITE FOx, functionalized with EV biomarker specific primary antibodies are used to bind rEVs spiked in buffer samples by anti-CD9 for calibration, or bind breast cancer specific EV with anti-EpCAM from complex liquid samples as blood plasma: MCF7-EV spiked for assay development, and human volunteer samples versus a spiked plasma control for proof of concept. As signal amplification to overcome label free detection limits, a sandwich assay was applied with anti-CD63. With subsequent sandwich additions, this may allow for multiplexing of a limited number of biomarkers.
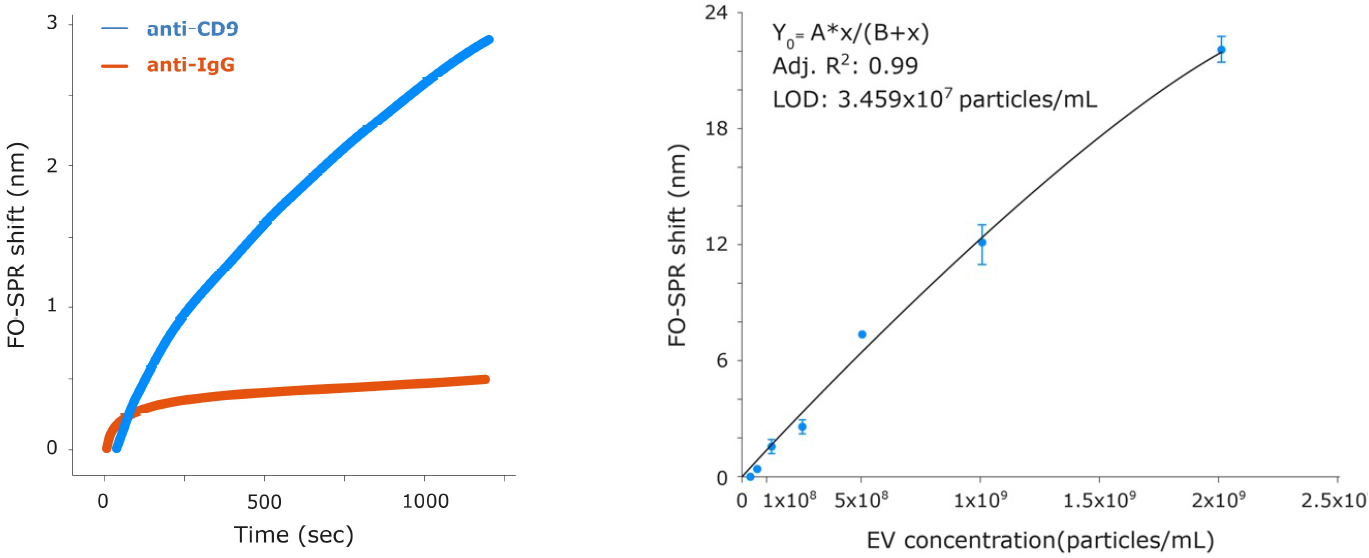
Figure 2. Quantification of rEV by sandwich assay. Left: Comparison of rEV (3×109 particles/mL) label-free binding specificity to probes functionalized with specific (anti-CD9) and non-specific (anti-IgG) antibodies by FO-SPR shift. Adapted from (1) Yildizhan et al. (2021). The rEV binding to anti-CD9 gave a signal six times greater than a negative control with anti-IgG. Right: FO-SPR shifts for a range of rEV concentrations down to 3.1x107 particles/mL using anti-CD63 / biotinylated anti-CD9 (Banti-CD9) capture/detection antibody pairs. Signal amplification was performed with anti-biotin conjugated gold nanoparticles (AuNP). Error bars represent standard deviation (n=3). Adapted from (1) Yildizhan et al. (2021).
Methods
FO-SPR was performed on WHITE FOx 96 well analytical instrument (FOx BIOSYSTEMS) and data were recorded with the FOx-SPR software to produce an open format .csv datafile. Analysis was performed with the FOx Dataprocessor preparing the files for MS- Excel, Graphpad Prism or TraceDrawer for concentration modeling.
Recombinant EVs (rEVs) were detected directly in DMEM cell medium supplemented with 10% exosome-depleted fetal bovine serum (ED-FBS) using anti-CD63 as capture antibody and Banti-CD9 as detection antibody. MCF7 EVs were analyzed using anti-EpCAM as capture antibody and Banti-CD9, Banti-CD63, Banti-CD81 or Banti-mix as detection antibody on 100-fold diluted plasma. For calibration curves and positive controls, EVs were spiked in the 10% FBS-supplemented cell culture medium or in 100 x diluted pooled plasma from healthy donors, respectively.
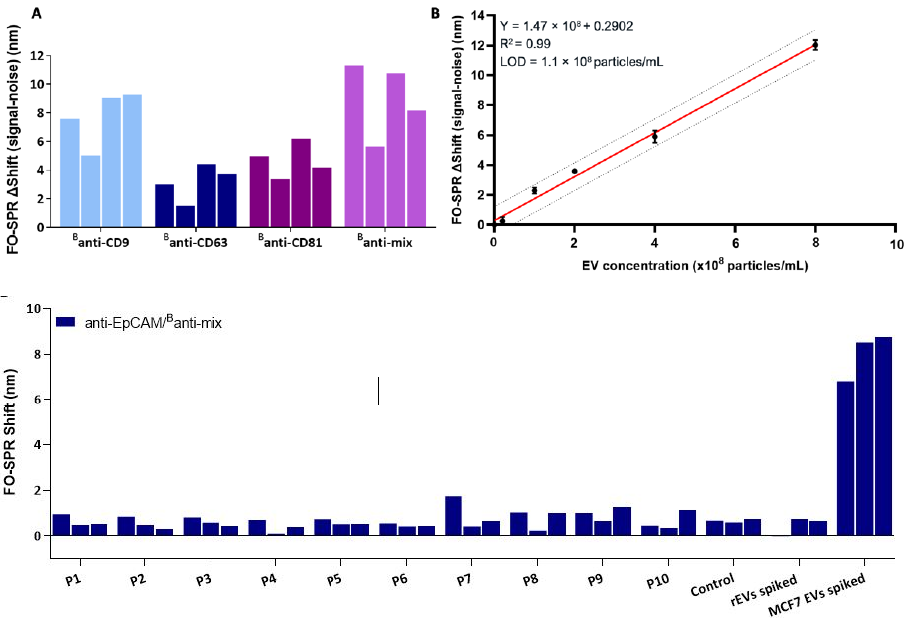
Figure 3. Detection of Breast cancer specific EVs in blood plasma. (A) FO-SPR sandwich bioassay with different antibody combinations for detecting spiked MCF7 EVs in 100-fold diluted pooled plasma. Bar graphs represent the FO-SPR shifts (after subtracting the negative control, i.e., SPR signal for 0 particles/mL) obtained from four independent measurements by combining anti-EpCAM capture antibody with different detection antibodies (Banti-CD9, Banti-CD63, Banti-CD81 or Banti-mix) for detecting MCF7 EVs at 1 × 109 particles/mL concentration. (B) FO-SPR-based detection of a series of MCF7 EV concentrations when using anti-EpCAM/Banti-mix antibody combination in spiked 100-fold diluted pooled plasma (obtained after sub- tracting the negative control, i.e., SPR signal for 0 particles/mL). Simple linear regression fitting was performed by GraphPad Prism software. The dotted lines indicate the 95% prediction bands for a new observation. Error bars represent one standard deviation (n = 3). (C) Specificity testing of the FO-SPR bioassays in 100-fold diluted plasma using anti-EpCAM/Banti-mix antibody combinations. P1 to 10 are plasma samples of 10 individual healthy donors unknown to be diagnosed with breast cancer. Control is 100-fold diluted pooled plasma. Two additional controls represent rEVs and MCF7 EVs spiked in 100-fold diluted pooled plasma. For each sample 3 independent replicate measurements were made. Specific Extracellular Vesicle detection and isolation in complex samples using FO-SPR. Adapted from (2) Yildizhan et al. (2023)
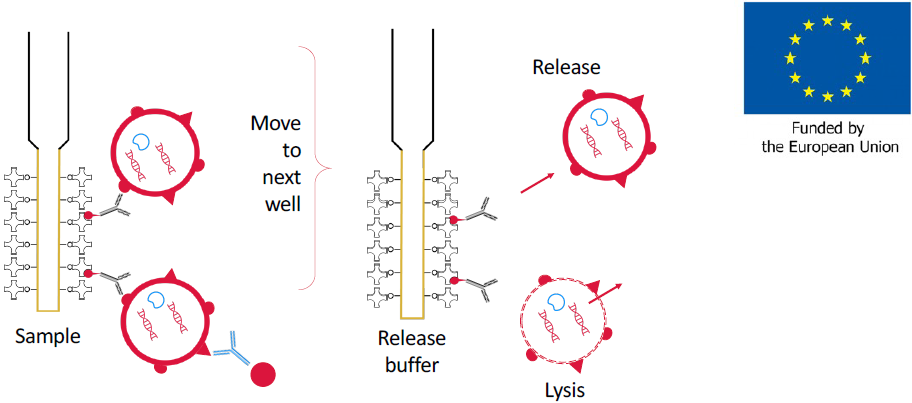
Figure 4. Development of automated isolation procedure. The captured EVs can be transferred to a well with release buffer to dissociate the EV from the probe, or to wells with lysis buffer to release their content for further analysis of their membrane proteins, cytoplasmic proteins, RNA, DNA and metabolites. This is supported with an EIC accelerator grant (*) to develop EV affinity FO-SPR sensor probes and analytical procedures for isolation and detection of biomarkers. The combined methodology aims to detect, quantify, isolate and characterize EVs for the early and specific diagnosis of disease markers direct from liquid biopsies such as blood. Adapted from FOx-EV EIC accelerator grant (*)
Conclusions
The reliable detection and quantification of EVs is becoming increasingly desirable due to their potential in disease diagnosis. However, their heterogeneity and the complexity of the matrices in which they are found, together with a lack of reference EV for assay development and the bias introduced by time-consuming and difficult-to-scale sample purification steps, cause difficulties for many bioanalytical methods.
FO-SPR provides a unique opportunity to detect EVs directly in complex matrices with minimal processing, thanks to its dip-in configuration. Here, we describe a FO-SPR assay capable of detecting EVs with LODs 103 times lower than the expected physiological concentration of EVs in healthy human plasma and 104 times lower than EV concentration in the plasma of cancer patients (3). FO-SPR, therefore, has great potential for sensitive EV analysis, particularly for analyzing EV subpopulations with specific biomarkers, which are typically at a much lower concentration in patient samples.
We are expanding this capability with direct isolation from crude liquid biopsies for further analysis of the EV content. Quantification of captured EV by FO- SPR can provide the necessary reference calibration to enable fast and reliable detection of biomarkers in patient samples. (*) Kris Ver Donck, Filip Delport | FOx BIOSYSTEMS NV, Agoralaan Abis, 3590 Diepenbeek March 2023.
Acknowledgement
This poster is based on the publications of Yildizahn et al. (2021) (1), Yildizahn et al. (2023) (2) and on the FOx-EV EIC accelerator grant (*) of FOx BIOSYSTEMS NV.
* Funded by the European Union. Views and opinions expressed are, however, those of the author(s) only and do not necessarily reflect those of the European Union or EIC. Neither the European Union nor the granting authority can be held responsible for them.
The authors gratefully acknowledge the Biosensors research group at KU Leuven and their partners at KU LEUVEN: PharmAbs, Rega Institute, Department of Oncology, Laboratory of Lipid Metabolism and Cancer, Laboratory for Thrombosis Research KULAK campus; and at Ghent University: Laboratory of Experimental Cancer Research, VIB Center for Medical Biotechnology & Department of Biomolecular Medicine; for their elaborate research on FO-SPR applications in the references, which provided the basis of the method and results shared in this poster.
References
- Yildizhan et al. FO-SPR Biosensor Calibrated with Recombinant Extracellular Vesicles Enables Specific and Sensitive Detection Directly in Complex Matrices. J. Extracell. Vesicles 2021, 10, e12059. https://doi.org/10.1002/jev2.12059.
- Yildizhan et al. Detection of Breast Cancer-Specific Extracellular Vesicles with Fiber-Optic SPR Biosensor. Int. J. Mol. Sci. 2023, 24, 3764. https:// doi.org/10.3390/ijms24043764
- E. Geeurickx et al. (2019). The generation and use of recombinant extracellular vesicles as biological reference material. Nature Communication 10, 1–12.
Last Updated: Nov 18, 2024