Statins are recommended as a first-line therapy for the management of lipid disorders, particularly those associated with elevated low-density lipoprotein cholesterol.
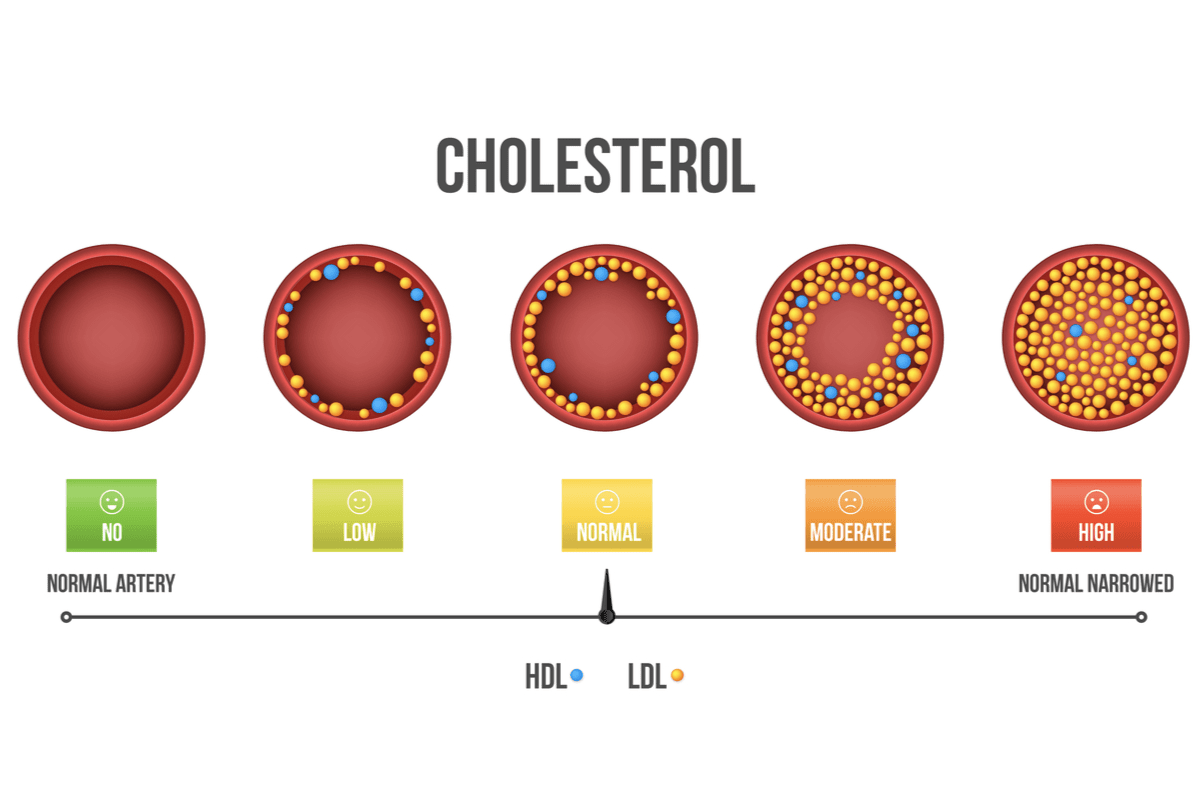
Image Credit: MIKHAIL GRACHIKOV / Shutterstock.com
In the 1950s and 1960s, it became obvious that elevated concentrations of plasma cholesterol represented a major risk factor for the development of heart disease, which led to the quest for drugs that could reduce it.
Since lovastatin had been commercialized, six statins including two semi-synthetic statins known as simvastatin and pravastatin, and four synthetic statins including fluvastatin, rosuvastatin and pitavastatin and atorvastatin, have been introduced to the market. The most popular statin today is atorvastatin.
The discovery of statins
While working at the Sankyo Company in 1976, the Japanese biochemist Akira Endo isolated a factor from the fungus Penicillium citrinum which he identified as a competitive inhibitor of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase). This substance, which he named compactin or mevastatin, was the first statin to be administered to humans.
Compactin was shown to lower plasma cholesterol in dogs, rabbits and monkeys. Nevertheless, some researchers remained skeptical, as compactin did not lower plasma cholesterol in rats, which was subsequently demonstrated to result from massive induction of HMG-CoA reductase in rat liver by inhibitors of the enzyme.
Clinical studies of compactin in Japan ensued soon after that, in addition to a number of other experimental studies around the world. In 1978, Alfred Alberts, along with his colleagues at Merck Research Laboratories, discovered a potent inhibitor of HMG-CoA reductase in a fermentation broth of Aspergillus terreus, which was named lovastatin, mevinolin or monacolin K. Conicidentally, Akira Endo independently identified the same compound within a year of Alberts’ discovery.
After animal safety studies were found to show no adverse effects, Merck began clinical trials of lovastatin in April of 1980. Still, this promising start was interrupted because the trials with structurally similar compactin were stopped by Sankyo Company in September of 1980, supposedly due to serious animal toxicity.
Image Credit: roger ashford / Shutterstock.com
Despite this finding, since the additional animal safety studies with lovastatin revealed no toxicity issues, in 1983 Merck decided to re-initiate the clinical development program, initially only in patients at very high risk of myocardial infarction.
The experience with lovastatin inspired efforts to chemically modify natural statins to make even more effective derivatives. To this end, researchers at Merck synthesized a side-chain ester analog from lovastatin, which is know widely known as simvastatin, with a 2.5-fold better activity in inhibiting HMG-CoA reductase activity.
Investigators at Warner-Lambert also synthesized a substituted H-pyrrole compound known as atorvastatin. This drug was approximately 3-4 times more potent in rat models when compared to lovastatin. Since then, several other statins including crilvastatin, nisvastatin and cerivastatin have also been synthesized by different pharmaceutical companies.
Entering the clinical use
On September 1, 1987, lovastatin became the first statin to be approved in the United States by the Food and Drug Administration (FDA). This agent is responsible for revolutionizing the treatment of hypercholesterolemia, initially achieving peak annual sales of more than $1 billion USD.
Simvastatin, an aforementioned side-chain ester analog of lovastatin, was approved for marketing in Sweden in 1988, with subsequent worldwide distribution. The approval and distribution of this drug were followed by pravastatin in 1991, fluvastatin in 1994, atorvastatin in 1997, cerivastatin in 1998, and rosuvastatin in 2003.
In 2012, the FDA introduced certain changes to the safety information on the labels of statins, which included a small increased risk of higher blood sugar levels and subsequent type 2 diabetes diagnosis. Furthermore, statin labels now also report potential cognitive effects such as confusion and memory loss.
References
Further Reading
Last Updated: Jan 18, 2023