3D printing refers to a computer-controlled construction system, in which material is deposited and layered in an organized manner to form a physical object based on a computationally designed plan. The ease of distribution of digital files that then allow one to fabricate an object locally could help to ease the burden faced by healthcare providers in the ongoing COVID-19 pandemic.
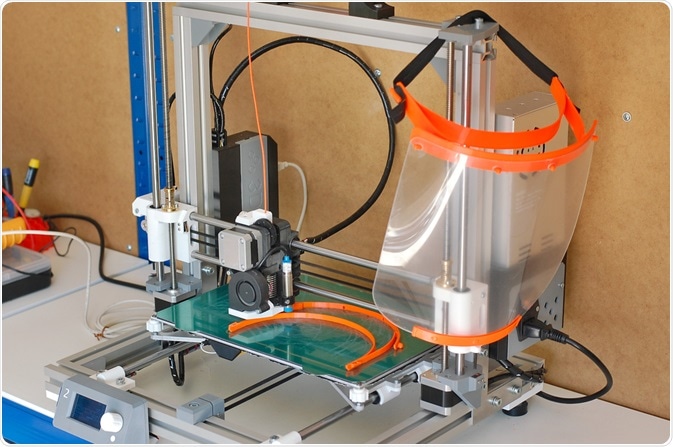 3D printing can be used to efficiently produce personal protective equipment (PPE) such as masks. Image Credit: Lucie Peclova / Shutterstock.com
3D printing can be used to efficiently produce personal protective equipment (PPE) such as masks. Image Credit: Lucie Peclova / Shutterstock.com
What is 3D printing?
3D printing has been around in some form for several decades, with early versions developed in the 1980s that could fabricate 3D models from photo-hardening plastics or from sheets of layered powdered metals.
At this time the technology failed to develop great interest and was mostly considered an alternative to metalworking processes that were more focused on the removal of material by milling or laser sintering, rather than adopting new additive processes (manufacturing by addition of metal, instead of by removal). It was not until the 2000’s that such processes for metalworking became more commonplace, and the 2010’s before suitable plastics were developed to allow quick 3D printing in this material.
Several methods of plastic 3D printing have been developed, one of the most readily available of which is fused filament fabrication. Using this technique, a continuous filament of thermoplastic material is fed into the printer where it is melted and applied to the 3D printed object in a layer-by-layer fashion.
How could 3D printing ease the COVID-19 burden?
The occurrence of the COVID-19 pandemic has burdened hospitals, care centers, and the public with an unprecedented demand for personal protective equipment and specific medical devices. Global supply chains have also been disrupted by the pandemic, making the urgent transportation of physical goods an additional challenge. Digital files suffer no such disruptions, and locally printed items could be an ideal solution to these problems.
The respiratory issues associated with COVID-19 left many in need of ventilator support, highlighting a massive shortage of suitable devices around the world. Several groups and companies responded with 3D-printable alternatives such as splitters that allow multiple patients to use a single ventilator machine, entirely 3D printable ventilators, and adaptors that allow commercially available snorkeling masks to be adapted for ventilation purposes, all of which can be printed and assembled on site.
Similarly, the demand for nasopharyngeal swabs was boosted massively at the start and throughout the pandemic, and several 3D printed options are now freely available to download and print.
The added customization enabled by 3D printing of personal items such as face masks allows well-fitting and comfortable products to be produced, enhanced by technologies such as 3D laser scanning that allow an exact model of a person’s face to be digitized and manipulated by computer-aided design. Additionally, 3D printed items are eminently recyclable, and can often be melted back down to their constituent polymers and re-used in 3D printing.
 Ventilators can be 3D printed. Image Credit: Dan Race / Shutterstock.com
Ventilators can be 3D printed. Image Credit: Dan Race / Shutterstock.com
Other less direct applications of 3D printing relevant to the pandemic include the production of training equipment for healthcare workers who are operating amongst an increasingly diminishing pool of trained personnel. For example, a 3D printed translucent training mannequin has been developed by a Singapore-based company that allows trainees to practice collecting nasopharyngeal swabs.
Temporary emergency shelters have also been 3D printed since before the pandemic, capitalizing on the fast and adaptable nature of 3D printing. In situations where hospitals are overloaded with patients and having difficulty isolating COVID-19 positive patients, such emergency shelters could be deployed in a timely manner, with the added advantage of easy transportation and redeployment if needed.
All devices intended for medical use must comply with a number of safety measures, from simple material toxicity to specific requirements concerning the function of a specialized device. ISO 10993 sets international standards for the biological evaluation of medical devices, to which most governing bodies generally comply, including the FDA.
This standard assesses sensitization and many aspects of toxicity when the device is in contact with various tissues from around the body, including intact or wounded skin, bone, and blood. 3D printed medical devices are no exception, and thus the responsibility for such testing may fall to the 3D file distributor, the end-user that prints the object, or the manufacturer of the filament of thermoplastic material with which the good was produced.
3D printed goods manufacturers may print and distribute the goods directly, and therefore have complete control of the materials used for manufacture, storage conditions, and distribution. Alternately, and in a way that best capitalizes on the advantages of 3D printing, they may simply sell the digital files that allow the customer to print the device themselves.
In this case, the manufacturer cannot be expected to control for the materials used or other conditions of production, passing these responsibilities to the customer. Intellectual property violation and copyright infringement is, and will likely continue to be, a dissuading factor for companies considering the distribution of digital goods, as digital files are easily copied and shared. However, many groups are happy to give away these potentially life-saving products for free during the pandemic.
References
- Ayyıldız, S. et al. (2020) 3D-Printed Splitter for Use of a Single Ventilator on Multiple Patients During COVID-19. 3D Printing and Additive Manufacturing, 7(4) https://www.liebertpub.com/doi/full/10.1089/3dp.2020.0102
- Clarke, A. L. et al. (2020) Coping with COVID‐19: ventilator splitting with differential driving pressures using standard hospital equipment. Anaesthesia, 75(7). https://associationofanaesthetists-publications.onlinelibrary.wiley.com/doi/full/10.1111/anae.15078
- Dhanani, J. et al. (2020) Increasing ventilator surge capacity in COVID 19 pandemic: design, manufacture and in vitro–in vivo testing in anaesthetized healthy pigs of a rapid prototyped mechanical ventilator. BMC Research Notes, 421. https://link.springer.com/article/10.1186/s13104-020-05259-z
- Callahan, C. J. et al. (2020) Open Development and Clinical Validation 2 Of Multiple 3D-Printed Sample-Collection Swabs: 3 Rapid Resolution of a Critical COVID-19 Testing Bottleneck. medRxiv. https://www.medrxiv.org/content/10.1101/2020.04.14.20065094v2
- Choong, Y. Y. et al. (2020) The global rise of 3D printing during the COVID-19 pandemic. Nature Reviews Materials, 5. https://www.nature.com/articles/s41578-020-00234-
Further Reading
Last Updated: Feb 11, 2021