The increased production of certain hormones throughout pregnancy is essential for maintaining the health of both the mother and fetus. Human chorionic gonadotropin hormone (hCG), human placental lactogen (hPL), progesterone, and estrogen are the most notable hormones involved in pregnancy.

Pregnancy. Image Credit: Ground Picture/Shutterstock.com
The placenta and estrogen
During the first ten weeks of gestation, both estrogen and progesterone are produced in the corpus luteum. Once the placenta is formed after implantation, this organ begins to produce these two steroid hormones at concentrations that gradually increase until reaching their peak in the last trimester. Soon after labor, both estrogen and progesterone levels drop significantly.
In addition to the placenta, other organs that secrete estrogen during pregnancy include the ovaries and, to a lesser extent, liver, muscle, bone, and brain.
Impact on the cardiovascular system
Once released into the bloodstream, estrogens will target various types of cells that express estrogen receptors, some of which are found in the endothelium, epithelium, muscle, bone, cartilage, hematopoietic cells, neurons, and glia. Within the cardiovascular system, estrogens exert pleiotropic effects that can begin very early in gestation.
Vasculature
Estrogens exert various effects on vasculature throughout pregnancy. More specifically, this steroid hormone directly acts on endothelium and vascular smooth muscle by participating in both rapid signaling pathways and genomic mechanisms.
Taken together, these actions contribute to the increased blood perfusion of maternal organs, including the uterus, during pregnancy. Additional vascular adaptations that are impacted by estrogen during pregnancy include the reduction in systemic vascular resistance by 25-30%, as well as the rise in cardiac output by 40%.
Cardiac hypertrophy
In addition to its involvement in vasculature during pregnancy, estrogen also has been associated with both pro- and anti-hypertrophic effects on the heart.
In terms of its anti-hypertrophic effects, estrogen has been shown to reduce the development of cardiac hypertrophy during pregnancy through its involvement in calcineurin degradation, control of phosphorylated p38 mitogen-activated kinase (MAPK) pathways, and regulating cardiomyocyte histone deacetylases. Notably, many of these effects have been observed in vitro at higher estrogen concentrations.
Comparatively, some of the pro-hypertrophic effects of estrogens during pregnancy have often been observed at lower concentrations. One observed pro-hypertrophic effect of the estradiol (E2) form of estrogen has been associated with a rise in extracellular signal-related kinase (ERK) activation.
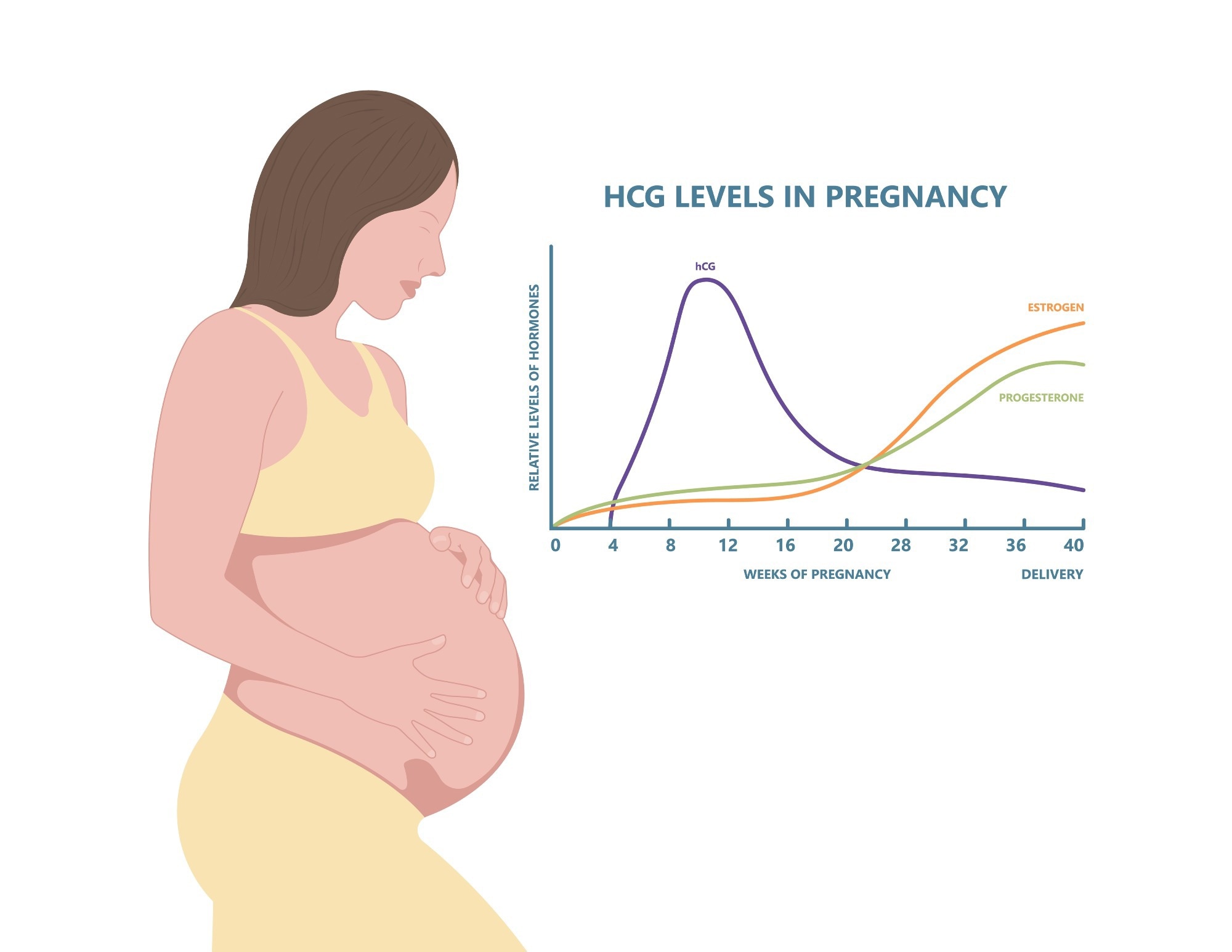
hCG levels in pregnancy. Image Credit: Pepermpron/Shutterstock.com
Preeclampsia
By definition, preeclampsia is a blood pressure condition that causes a pregnant person to have high blood pressure and high levels of protein in their urine. As one of the most common complications of pregnancy, preeclampsia is also a primary cause of both maternal and fetal mortality and morbidity.
Preeclampsia is considered a placental disease; however, there remains considerable debate regarding the precise pathophysiological mechanism responsible for this condition. The dysregulation of estrogen levels during pregnancy, as well as the enzymes involved in the biosynthesis of this steroid hormone, have been described during preeclampsia.
For example, several studies have observed low plasma levels of E2 in patients with both severe and mild preeclampsia, as well as in the placental tissue isolated from pregnant mothers with this condition. Additionally, one study found that pregnant women with preeclampsia had lower levels of E2 as compared to healthy pregnant women.
In addition to E2, researchers have also observed lower levels of the estrone (E1) form of estrogen in severe preeclampsia, as well as estriol (E3) in patients with both mild and severe preeclampsia.
The important role of estrogens in promoting placental angiogenesis and uterine artery vasodilation also supports the hypothesis that estrogen levels may contribute to the pathogenesis of preeclampsia. For example, E2 increases the synthesis of nitric oxide (NO) as well as the levels of various angiogenic factors like vascular endothelial growth factors (VEGF) and placental growth factor (PlGF). Thus, reduced E2 levels may have downstream effects on the levels of both NO and angiogenic factors, thus reducing vasodilation and angiogenic processes that can contribute to preeclampsia.
Gestational diabetes
Another common complication of pregnancy is gestational diabetes (GDM), which affects about one in seven pregnant women. One hypothesized factor involved in the development of GDM is altered E2 levels, as this form of estrogen contributes to β-cell function, which increases the synthesis of insulin. E2 is also involved in reducing the expression of the insulin-sensitive membrane transporter GLUT4, which may also contribute to the insulin resistance observed in GDM.
To compensate for the gradually increasing levels of estrogen throughout pregnancy that reduce the sensitivity of insulin during this period, the mother will secrete additional insulin. In the event that the added insulin does not sufficiently balance the resistance to insulin, blood glucose levels will rise and subsequently increase the mother’s risk of GDM.
References
- Hormones During Pregnancy [Online]. Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/staying-healthy-during-pregnancy/hormones-during-pregnancy.
- Kodogo, V., Azibani, F., & Sliwa, K. (2019). Role of pregnancy hormones and hormonal interactions on the maternal cardiovascular system: a literature review. Clinical Research in Cardiology 108; 831-846. doi:10.1007/s00392-019-01441-x.
- Napso, T., Yong, H. E. J., Lopez-Tello, J., & Sferruzzi-Perri, A. N. (2018). The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Frontiers in Physiology. doi:10.3389/fphys.2018.01091.
- Berkane, N., Liere, P., Oudinet, J., et al. (2017). From Pregnancy to Preeclampsia: A key Role for Estrogens. Endocrine Reviews 38(2); 123-144. doi:10.1210/er.2016.1065.
- Thibeault, A. H., Sanderson, J. T., & Vaillancourt, C. (2019). Serotonin-estrogen interactions: What can we learn from pregnancy? Biochimie 161; 88-108. doi:10.1016/j.biochi.2019.03.023.
- Choudhury, A. A., & Rajeswari, V. D. (2021). Gestational diabetes mellitus – A metabolic and reproductive disorder. Biomedicine & Pharmacotherapy 143. doi:10.1016/j.biopha.2021.112183.
Further Reading
Last Updated: Oct 7, 2022