Real-world applications in neuroimaging
Leading companies and research driving innovation
Challenges and considerations in implementing deep learning
Deep learning (DL) is a type of artificial intelligence (AI) that utilizes artificial neural networks (ANNs) to process data through two or more layers, each of which can recognize complex features of the data.
All deep learning models are built on fundamental computational units that take in inputs, process them, and produce outputs—either forwarding them to the next layer or using them as the final result.1
Recent advancements in the technological capabilities of central processing units (CPUs), graphics processing units (GPUs), and learning algorithms, combined with the availability of big data, have allowed DL to become one of the most powerful AI technologies.
As a result, DL has been incorporated into various applications, some of which include computer vision, natural language processing, and speech improvement.
DL has also been widely used to support the analysis of medical images, which can include the classification, detection/localization, registration, and segmentation of medical images.2
To date, several studies have reported the use of DL for analyzing brain, chest, eye, breast, cardiac, abdomen, and musculoskeletal imaging.
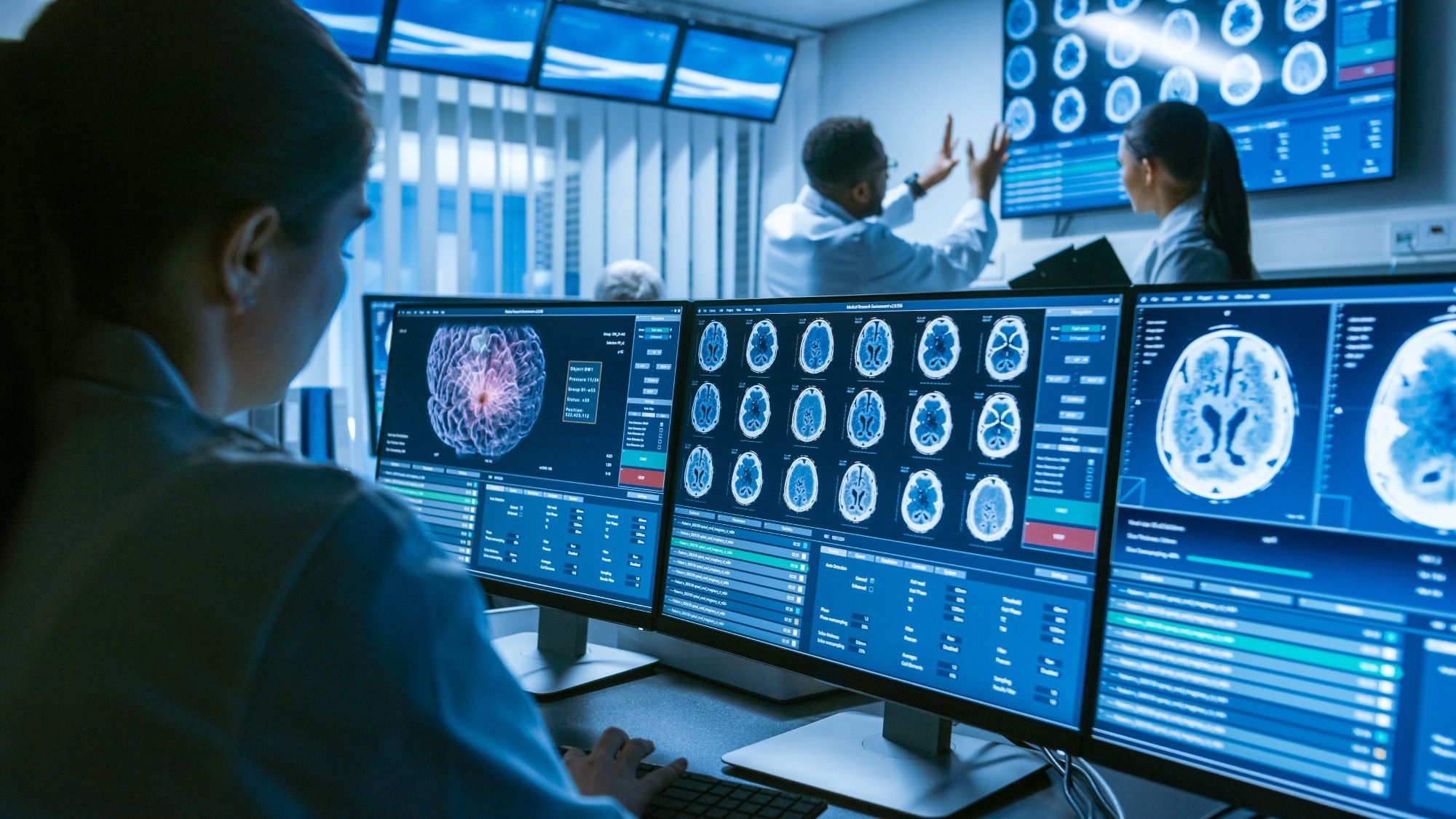 Image Credit: Gorodenkoff/Shuterstock.com
Image Credit: Gorodenkoff/Shuterstock.com
Real-world applications in neuroimaging
Disease detection
Deep learning (DL) has been extensively studied for its potential to enhance the sensitivity of medical imaging in detecting various neurological disorders. These include Alzheimer’s disease, Parkinson’s disease, autism spectrum disorder (ASD), schizophrenia, brain tumors, and multiple sclerosis (MS).
Beyond diagnosis, AI and DL also offer valuable insights into how patients are responding to treatment—information that can help clarify prognoses and guide the personalization of future care strategies.
Convolutional neural networks (CNNs), a key subclass of DL models, are particularly well-suited for medical imaging tasks. CNNs leverage spatial information from neighboring pixels (in 2D) or voxels (in 3D), which are processed through a series of convolutional layers to generate multiple feature maps.
For Alzheimer’s disease, both 2D and 3D CNN-based approaches have been trained on magnetic resonance imaging (MRI) data for image segmentation and classification. CNNs stand out for their ability to automatically learn and refine features from raw imaging data, making them a strong fit for diagnostic analysis.
In addition to CNNs, other DL architectures such as autoencoders (AEs) and recurrent neural networks (RNNs) have been explored—particularly in the context of ASD diagnosis.
AE-based methods use variations or stacked architectures to extract discriminative representations from imaging data. Meanwhile, RNN-based approaches have been evaluated for their capacity to process resting-state functional MRI (rs-fMRI) data from ASD patients, leveraging their strength in handling temporal patterns in brain activity.
Stroke and trauma assessment
Traditional medical imaging can be time-consuming, which poses a challenge during medical emergencies such as stroke or other neurological trauma. In these situations, clinicians may opt for lower-resolution scans to speed up image acquisition. However, the trade-off is image quality—these lower-resolution images may lack the detail needed for accurate diagnosis. Deep learning (DL) methods offer a promising solution by reconstructing raw, low-quality data into high-resolution images, helping to preserve diagnostic accuracy even under time constraints.
DL can also help address the high costs associated with MRI and other diagnostic imaging techniques. By accelerating image acquisition without introducing additional artifacts, DL has the potential to make advanced imaging more efficient and accessible, without compromising quality.3
Can AI Outperform Doctors in Diagnosing Infectious Diseases?
Leading companies and research driving innovation
The Center for Clinical Data Science at Massachusetts General Hospital (MGH) and Brigham and Women’s Hospital have released DeepNeuro as an open-source tool for public use. DeepNeuro, which has been trained on a large dataset of neuroimaging studies, is a Python-based deep learning (DL) framework designed to streamline the training and evaluation of DL models on new medical imaging data.
Users can directly load their datasets and take advantage of pre-built training scripts, making it a valuable resource for both research and educational purposes.
Beyond ease of use, DeepNeuro offers several practical advantages. It can be integrated with other machine learning (ML) software to support method ensembling, and it can be incorporated into diagnostic systems to enhance usability and performance in clinical workflows.4-5
Another notable tool in this space is NeuroQuant, an FDA-approved ML software designed to analyze MRI brain scans. NeuroQuant provides precise, quantitative measurements of brain structure volumes—data that can help detect and track pathological changes associated with conditions such as Alzheimer’s disease, multiple sclerosis (MS), and other serious neurological disorders. By offering clear, standardized metrics, NeuroQuant enables radiologists to deliver more consistent and objective assessments of brain tissue.5
Ethical Considerations in AI-Driven Healthcare
Challenges and considerations in implementing deep learning
Despite the many advantages of deep learning (DL) in neuroimaging analysis, its clinical adoption is still in the early stages. One major limitation is overfitting, which can occur when complex models are trained on small datasets, leading to poor generalizability. To address this, the use of large, labeled, and publicly available medical imaging datasets is essential for improving the reliability and robustness of DL applications.
In addition to data-related challenges, ethical and legal concerns—along with the difficulty of interpreting DL results in psychological or mechanistic terms—can make some clinicians and stakeholders hesitant. The "black box" nature of many DL models adds to the uncertainty, especially in high-stakes medical decision-making.
DL algorithms also require substantial amounts of high-quality data for training, which presents a hurdle when studying rare neurological disorders where data are limited. To mitigate this, researchers have developed data augmentation techniques to synthetically increase the number of training samples and improve model performance.
While AI and DL can be valuable tools in supporting clinical diagnoses, they are not a replacement for clinical judgment. Treatment decisions must continue to center around the physician’s expertise, guided by a comprehensive understanding of the patient’s unique needs.
References
- Avbersek, L. K., & Repovs, G. (2022). Deep learning in neuroimaging data analysis: Applications, challenges, and solutions. Frontiers in Neuroimaging 1. doi:10.3389/fnimg.2022.981642.
- Zhang, L., Wang, M., Liu, M., & Zhang, D. (2020). A Survey on Deep Learning for Neuroimaging-Based Brain Disorder Analysis. Frontiers in Neuroscience 14(779). doi:10.3389/fnins.2020.00779.
- Zhu, G., Jiang, B., Tong, T., et al. (2019). Applications of Deep Learning to Neuro-Imaging Techniques. Frontiers in Neurology 10. doi:10.3389/fneur.2019.00869.
- Beers, A., Brown, J., Chang, K., Hoebel, K., et al. (2022). DeepNeuro: an open-source deep learning toolbox for neuroimaging. Neuroinformatics 19(1); 127-140. doi:10.1007/s12021-020-09477-5.
- “NeuroQuant” [Online]. Available from: https://americanhealthimaging.com/services/neuroquant/.
Further Reading
Last Updated: Apr 7, 2025