Introduction
Types of medical alert device
The problem associated with the complexity of many medical devices
Revaluating approaches to the medical alert device problem
References
Further reading
Medical alert devices are medical technologies that tend to the complex physiological monitoring of patients. This may include the continuous infusion of medication and enables invasive treatments to occur, such as mechanical ventilation, renal replacement therapy, and extracorporeal life support. Typically, each medical device comes with a standardized set of reporting tones and outputs.
 Image Credit: Peter Titmuss/Shutterstock
Image Credit: Peter Titmuss/Shutterstock
Types of medical alert device
Many types of medical alert devices can transmit vital signs and other data to healthcare professionals, which enable them to finetune treatment approaches. Patients can also transmit biometric data to healthcare professionals through the use of wearables or remote monitoring devices.
These include pulsometers and blood pressure cuffs. Devices facilitate the movement towards telemedicine-based healthcare which has increased in popularity/utility since the pandemic, as these technologies can access patient information from a dashboard or clinical decision support system, subsequently compile the data, and display patient status close to real-time.
The problem associated with the complexity of many medical devices
However, due to the complexity of modern patient care, patients may be connected to multiple medical devices simultaneously, and each individually sounds alarms without prioritization or coordination with other medical devices attached to the patient. This may result in a series of uncontrolled alarms in the context of a noisy and distracting environment in an operating theatre or an intensive care unit.
This problem has been documented in the literature. For example, according to one study of medical devices reported in a single hospital in the united states with 77 intensive care beds, over two million unique physiological alarms were recorded in a single month.
Other estimates suggest that each clinician in an intensive care unit is subjected to between 100 and 120 medical device alarms throughout every eight-hour shift. It is believed that noise and distraction can produce fatal consequences for patients. In quantitative terms, between 2005 and 2010, 566 alarm-related patient deaths were reported to the United States Food and Drug Administration.

 Related: What are Medical Devices?
Related: What are Medical Devices?
Revaluating approaches to the medical alert device problem
In a paper published in the British Journal of Anesthesia in 2021, Roche et al. evaluated innovative approaches to tackling the problem of alarms in healthcare using auditory icons and human voice alerts. An auditory icon is defined as a sound that is associated with an action or event; for example, the sound of crunching paper when a file is deleted. Auditory icons are considered to be the recommended alarm sound across seven standardized risk categories in the International Electrotechnical Commission (IEC) on auditory displays for medical electrical equipment.
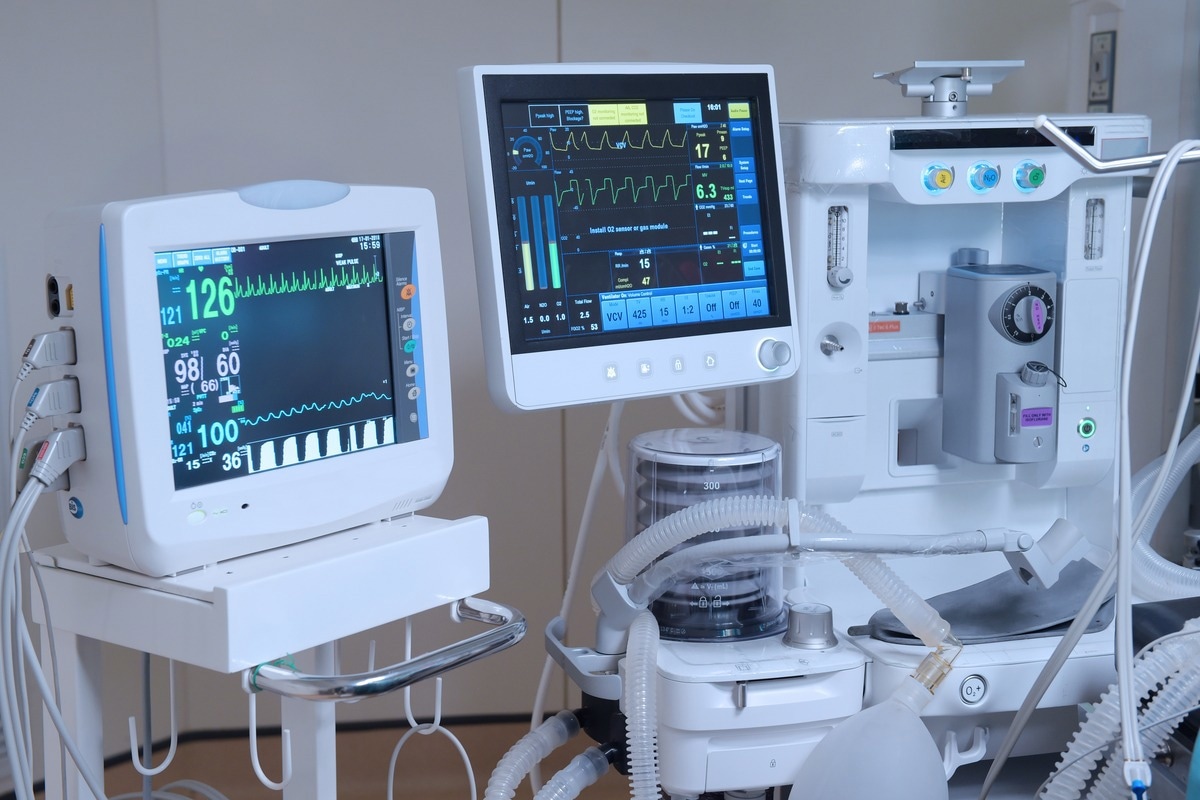 Image Credit: nimon/Shutterstock
Image Credit: nimon/Shutterstock
Roche et al. used these risk categories recommended in the IEC standard, along with different auditory sounds. Alongside these, Roche used voice notifications; these have been employed since the 1990s but there has been concern about their utility for clinical notification. The paper represents the first comparison of auditory icons and voice notification (complete with tone-based alternatives).
Both voice alerts and auditory icons can be combined to communicate all relevant information about the triggering event. This enables the alarm output to be more informative; an example of this is a voice notification of ‘temperature high’ or the sound of fire as indicators of a potential fever as opposed to indiscriminate beeps or tones.
Investigators reported that the participants were able to identify a significantly higher proportion of notifications using voice alerts (98%) and auditory icons (54%) relative to the state-of-the-art device alarms.
Between voice alerts and auditory icons, voice alerts were much more successful, with participants correctly identifying the action 58 times more correctly as compared to auditory icons. In addition, participants were more confident and quick in their ability to perceive the meaning of the alert. Principally, this was achieved by making the alert more complex and meaningful – using a human voice that conveyed the reason for the concern.
The researchers proposed that although the complexity of the alert output is increased, it enhances comprehensibility, and decreases the burden on healthcare professionals as they do not need to rely on memorizing arbitrary sounds and distinct medical issues can be instantly identified and communicated (i.e, instant differentiation).
 Image Credit: Mascha Tace/Shutterstock
Image Credit: Mascha Tace/Shutterstock
References
- International Electrotechnical Commission. Medical electrical equipment—Part 1–8: general requirements for basic safety and essential performance—collateral standard: general requirements, tests, and guidance for alarm systems in medical electrical equipment and medical electrical systems. IEC60601-1-2006/AMD2:2000 2020. Available from: https://www.iso.org/standard/41986.html. (Accessed January 2022).
- Edworthy JR, McNeer RR, Bennett CL, et al. (2018) Getting Better Hospital Alarm Sounds Into a Global Standard. Ergonomics in Design. doi:10.1177/1064804618763268
- Roche TR, Braun J, Ganter MT, Meybohm P, Herrmann J, Zacharowski K, Raimann FJ, Piekarski F, Spahn DR, Nöthiger CB, Tscholl DW, Said S. Voice alerting as a medical alarm modality for next-generation patient monitoring: a randomised international multicentre trial. Br J Anaesth. 2021 Nov;127(5):769-777. doi: 10.1016/j.bja.2021.07.015. Epub 2021 Aug 26. PMID: 34454710.
- Webster CS, Sanderson P. (2021) Need for a new paradigm in the design of alarms for patient monitors and medical devices. Br J Anaesth. doi: 10.1016/j.bja.2021.08.001.
- Haleem A, Javaid M, Singh RP, et al. (2021) Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens Int. doi: 10.1016/j.sintl.2021.100117.
Further Reading
Last Updated: Jul 4, 2022