Human cells are equipped with several mechanisms with which they can stem the spread of viral infections throughout the body.
Zinc-finger antiviral proteins (ZAP) are produced by both human and animal cells to stop the spread of viruses by targeting viral mRNA, which can lead to innate immune mechanisms against infections.
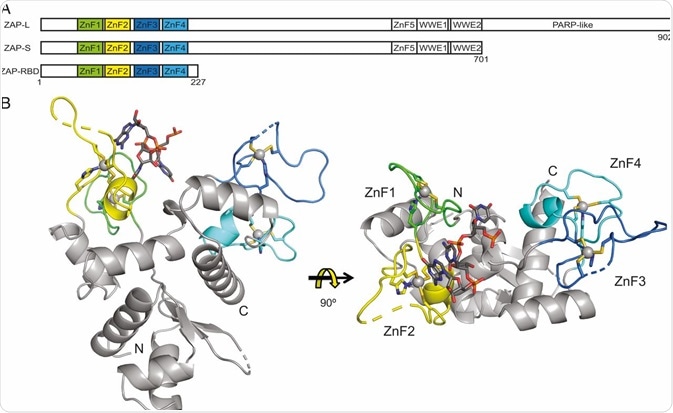
Image Credit: pnas.org
What is a zinc-finger (ZNF)?
A zinc-finger is a protein structural motif that can be found in many proteins and possess a wide range of molecular functions, playing a role in the regulation of numerous cell processes.
A zinc-finger protein features one or more zinc ions that stabilize the peptide fold by holding together its structural elements. ZNFs can interact with both DNA and RNA, as well as poly-ADP-ribose (PAR), among other proteins.
Zinc fingers in disease
ZNFs have been implicated in several human diseases. It is important in the progression of cancer, including the initial formation of cancer, to its metastasis in a secondary site in the body, but it has also been found to be downregulated in its expression, which may inhibit tumor progression.
Additionally, there is building evidence to suggest that ZNFs could play an important role in the inflammatory skin condition psoriasis. Other diseases that may be characterized by ZNF functionality include congenital heart diseases and diabetes.
Zinc-finger antiviral proteins in disease restriction
The expression of the zinc-finger antiviral protein has been shown to inhibit and restrict several different viral infections, including viruses from the retrovirus, alphavirus, filovirus, and hepadnavirus families.
The alphaviruses that ZAP has been found to significantly inhibit include the Sindbis virus, Ross River virus, and the Venezuelan equine encephalitis virus.
ZAP can specifically inhibit the replication of the Sindbis virus by stopping the accumulation of viral RNA in the cytoplasm. In addition to the Sindbis virus, ZAP also specifically inhibits the Moloney murine leukemia virus (MLV), a retrovirus that can cause cancer in mice and other rodents.
In humans, low expression of zinc-finger antiviral proteins has shown to be closely linked with disease progression and poor outcomes for people diagnosed with hepatocellular carcinoma (HCC), the most common type of primary liver cancer.
The zinc-finger antiviral protein can fight the influenza virus among other viral infections because it is encoded by the ZC3HAV1 gene, which facilitates action against viral infections. Signaling proteins known as interferon are released by infected cells to increase the antiviral responses of other uninfected, nearby cells.
Zinc-finger antiviral proteins can detect and deplete RNA viruses because it has high amounts of CG dinucleotides. It has been suggested that viral RNA recognition by ZAP is made possible by pockets on the protein surface of RNA only being able to accommodate a CG dinucleotide, but the full functionality of zinc-finger antiviral proteins is not known.
The portions of ZAP that have been identified include an N-terminal ∼227-aa RNA-binding domain (RBD) that features four CCCH zinc-fingers. This is sometimes called N-ZAP and is thought to be necessary for the protein’s antiviral capabilities.
Studies have used site-directed mutagenesis to disrupt each individual zinc-finger to determine each motif’s importance in ZAP’s antiviral activity. It was found that disrupting the second (C88R) or fourth (H191R) zinc-finger almost entirely stopped the ZAP’s ability to inhibit viral replication while disrupting the first (H86K) or third (C168R) finger only resulted in mild impairment of ZAP’s antiviral capabilities.
Although the mechanisms by which ZAP inhibits viral replication are understood for certain viruses, such as the Moloney murine leukemia virus, it is not understood how ZAP inhibits other types of virus. There is further research needed to ascertain which mechanism is responsible for facilitating the downregulation of mRNAs by zinc-finger antiviral proteins.
Image Credit: Kateryna Kon/Shutterstock.com
SARS-CoV-2 and zinc-finger antiviral proteins
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the coronavirus responsible for causing the severe respiratory illness COVID-19, the initial outbreak of which was in December 2019, leading to a global pandemic. All current treatments for COVID-19 are supportive, and so investigations into therapeutic drugs for the novel coronavirus are key.
A scientific report has suggested that ZAP can restrict SARS-CoV-2. Interferon types I, II, and III were found to significantly restrict SARS-CoV-2 and brought on the expression of zinc-finger antiviral proteins to fight the virus. This discovery, which has not been proved conclusive as of yet, may be important in the development of combination therapies for COVID-19.
Summary
Zinc-finger antiviral proteins are one of how human cells can stop the spread of viruses throughout the body. Although their mechanisms are not fully understood, ZAPs are a potential area of study for the development of therapeutic drugs for a wide range of viruses.
Sources
- Bick, M.J., et al. (2003). Expression of the zinc-finger antiviral protein inhibits alphavirus replication. Journal of Virology. https://jvi.asm.org/content/77/21/11555.short
- Bieniasz, P.D., et al. (2019). Structure of the zinc-finger antiviral protein in complex with RNA reveals a mechanism for selective targeting of CG-rich viral sequences. PNAS Microbiology. https://doi.org/10.1073/pnas.1913232116
- Cai, J. et al. (2018). Association of low zinc-finger antiviral protein expression with progression and poor survival of patients with hepatocellular carcinoma. Cell Physiology Biochemistry. https://doi.org/10.1159/000493285
- Carroll, M.R., et al. (2004). The zinc-finger antiviral protein directly binds to specific viral mRNAs through the CCCH zinc-finger motifs. Journal of Virology. https://jvi.asm.org/content/78/23/12781#sec-12
- Cassandri, M., et al. (2017). Zinc-finger proteins in health and disease. Cell Death Discovery. https://www.nature.com/articles/cddiscovery201771.
- Nchioua, R. et al. (2020). SARS-CoV-2 Is Restricted by Zinc Finger Antiviral Protein despite Preadaptation to the Low-CpG Environment in Humans. American Society for Microbiology. https://doi.org/10.1128/mBio.01930-20
- Protein Data Bank. (2007). https://pdb101.rcsb.org/motm/87
Further Reading
Last Updated: Mar 4, 2021