According to the US Centres for Disease Control and Prevention (CDC), dracunculiasis, also referred to as Guinea worm disease, is a vector-borne parasitic disease caused by the Dracunculus medinensis roundworm.
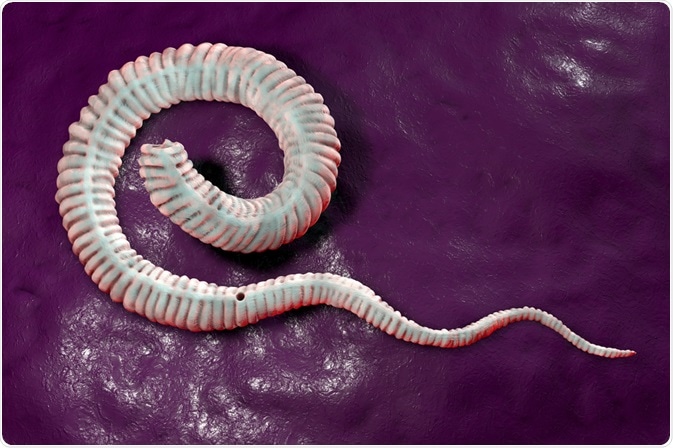 Image Credit: Kateryna Kon / Shutterstock.com
Image Credit: Kateryna Kon / Shutterstock.com
Limited to remote, rural African countries without access to clean drinking water, it is transmitted exclusively through the consumption of contaminated water. Those who become infected remain asymptomatic for approximately one year.
Upon onset of symptoms, the patient develops a painful blister, usually on the leg or foot, accompanied by burning pain, swelling and vomiting which burst, releasing the adult worm.
What causes dracunculiasis?
A person becomes infected after drinking water that contains copepods, small water fleas infected with Dracunculus medinensis larvae. Once ingested, the copepod is dissolved by stomach acid, releasing the larvae which migrate through the host intestine into the abdominal cavity.
After approximately three months, the larvae mature and copulate, after which the male worm dies and is reabsorbed by the host body. The female worm, which can grow up to 100cm, migrates to subcutaneous tissue to fully mature into the adult worm.
Approximately one-year post-infection, the gravid adult worm begins to migrate to the host’s skin surface, penetrating the dermis and causing an inflammatory reaction. This results in a characteristic blister usually found on the lower extremities but occasionally found on the hand or scrotum, which ruptures within 24 – 72 hours.
Upon rupture, the adult worm slowly begins to emerge from the resultant ulcer over several days, accompanied by extreme burning pain, swelling, dizziness and vomiting. When the host submerges the affected area in water, the gravid female releases a milky white liquid containing thousands of larvae thus completing the lifecycle of the parasite.
The prevalence of dracunculiasis is closely correlated with rainfall. In dry areas, transmission increases during rainy periods when surface water is available. In wet areas, transmission increases during the dry season, when sources of drinking water are more limited. Transmission sites are commonly areas of stagnant water such as ponds and wells.
Complications associated with dracunculiasis
Although the disease is not fatal, it causes significant morbidity. Infected patients can be unwell and in significant pain, reducing their mobility for several months and leading to a significant economic burden on affected villages.
Secondary complications are usually associated with the risk of infection to the affected area and include cellulitis, sepsis and arthritis. As access to health care in affected areas may be limited, secondary complications from infection may lead to lifelong disability or death.
Treatment and prevention
There are no medicines to treat dracunculiasis and no vaccine to prevent infection, although anti-inflammatory drugs may be used to soothe the ulcerated areas and facilitate worm removal.
Analgesics can be used to treat localized pain, and antibiotic ointments and occlusive dressings can reduce the risk of secondary infections. Medications that have been shown to be effective at treating other similar parasitic infections have been shown to increase the risk of anomalous worm migration, leading to exit blisters in areas other than the leg or foot.
Once the worm begins to emerge from the wound, the rest must be gradually removed by a few centimeters each day by winding it slowly around gauze or stick. Gentle massaging of the area can help loosen the worm, but the process may take several weeks.
Care must be taken not to break the worm during removal, as the remaining section of the dead worm will decay inside the body, causing bacterial infection.
In the absence of efficacious treatments, the most effective strategy against dracunculiasis is the prevention of infection. In the early 1980s, an advocacy initiative to eradicate dracunculiasis was launched by the CDC. By 1991, the World Health Organisation declared a commitment to eradicate the disease globally by 1995.
The first national campaign began in India in 1982 and was quickly followed by all other known countries of endemicity. This has had a remarkable impact with a 98% reduction in reported cases between 1986 and 2000.
Several interventions were implemented, and these included:
- The provision of safe water supplies, including the maintenance of new water supplies;
- Developing effective tracking surveillance methods to detect new cases within 24 hours of worm emergence;
- Preventing the transmission of larvae whilst the worm is expelled from the body by adequate wound treatment and use of occlusive dressings;
- Preventing release of larvae by preventing infected people from submerging in water sources, and encouraging them to immerse emerging worms in buckets of water to later be emptied on dry land;
- Filtering water before drinking to remove infected copepods;
- Employing more aggressive vector control strategies by treating water bodies with larvicide to kill copepods;
- Delivering targeted programs of health education and behavioral change, particularly in the most rural areas.
The most successful interventions were those aimed at education and behavior change, particularly those that promoted the use of nylon mesh fabric to filter copepods from drinking water. All national programs now donate such filters to affected communities.
Additionally, providing clear education regarding the transmission of the disease to reduce patient contact with ponds and other bodies of water has been shown to reduce new cases by 88%, even in the absence of available filter cloth.
References
Greenaway C. (2004). Dracunculiasis (guinea worm disease). CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne, 170(4), 495–500.
Gulanikar A. (2012). Dracunculiasis: two cases with rare presentations. Journal of cutaneous and aesthetic surgery, 5(4), 281–283. https://doi.org/10.4103/0974-2077.104918
Muller R. (1979). Guinea worm disease: epidemiology, control, and treatment. Bulletin of the World Health Organization, 57(5), 683–689.
Sullivan, J. J., & Long, E. G. (1988). Synthetic-fibre filters for preventing dracunculiasis: 100 versus 200 micrometres pore size. Transactions of the Royal Society of Tropical Medicine and Hygiene, 82(3), 465–466. https://doi.org/10.1016/0035-9203(88)90164-2
Further Reading
Last Updated: Dec 23, 2020