Skip to:
Glioblastoma multiforme (GBM) is one of the most aggressive brain cancers (gliomas) that arises from glial cells (astrocytes and oligodendrocytes) within the brain. GBM is a grade IV tumor, the most aggressive form of cancer. Patients typically die within 15 months of diagnosis, though surgery and newer treatments may improve prognosis.
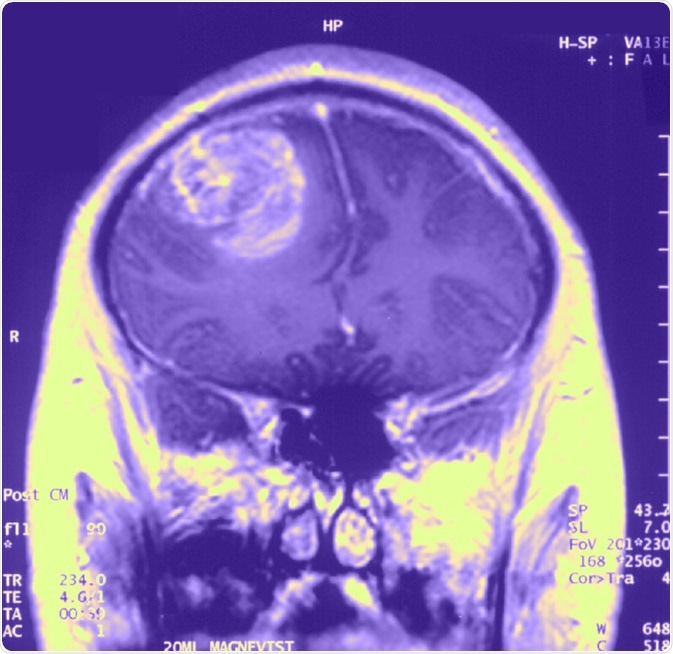
Gliobastoma (astrocytoma) WHO grade IV - MRI coronal view, post contrast. 15 year old boy. image Credit: Christaras A
Prevalence & Incidence
About 3 per 100,000 people develop GB each year, though the new number of GBM cases have almost doubled within the UK between 1995 and 2015. After meningioma, GBM is the second most common form of brain cancer. The prevalence of GBM is more common amongst males than females, and usually begins around 64 years of age.
Symptoms
The symptoms of GBM vary depending on where the tumour has arisen, and may not actually present until the tumour has grown to a large size, but general symptoms include:
- Constant Headaches
- Seizures
- Memory Loss
- Nausea and Vomiting
- Double or Blurred Vision
- Personality & Mood Changes
- Increasing Speech Difficulties
Causes
Most cases of GBM occur de novo with unknown causes, though about 5-10% progress from low-grade astrocytoma. Genetics may also play a part in the onset of GBM, with risk factors for Li-Fraumeni syndrome or neurofibromatosis may make the likelihood of developing GBM. Certain viral infections such as SV40 and HHV-6 may also increase the risk of developing GBM.
As the name suggests, glioblastoma is multiforme grossly, microscopically and genetically. Grossly the tumour contains regions of necrosis and haemorrhages. Microscopically, there is microvascular proliferation, pleomorphic cells and pseudopalisading necrosis. Genetically, the tumours contain numerous deletions, amplifications as well as point mutations that affect cellular signalling pathways including EGFR, PDGFR, loss of p53, CDK4 amplification and Rb loss. It is estimated that due to the heterogeneity of these diverse tumours, a tumour of 109 cells may contain as many as 106 cells with mutations.
Diagnosis
Diagnosis is usually made by performing neuroimaging to localize the tumour. These tools include CT scans, PET scans and MRI scans. Once a tumour has been located, a biopsy is taken through a craniotomy for definitive diagnosis.
The biopsy sample allows neuropathologists to grade the tumour, classify the tumor type, and assess cell type involvement (e.g. astrocytoma) as well as signs of metastasis or whether the tumour is primary or secondary. This is important as primary and secondary GBMs have different characteristics and respond differentially to treatment strategies as well as affecting prognosis.
Treatments
At present, there is no known method to prevent GBM. The most common treatment strategy is surgical removal of the primary tumour followed by radiotherapy or chemotherapy. Generally, removal of up to 98% of tumour can significantly improve prognosis, and the greater the extent of tumour removal, the better the prognosis.
However, most treatment strategies are severely complicated due to the nature of GBMs. For example, the perimeter of the tumour is muddled by infiltrating and migrating cells which makes it next to impossible to fully remove the tumour.
Depending on the location of the tumour, access through the skull/brain may be difficult, and also the risk of damaging important brain regions may be high. Therefore, the entirety of the tumour may not always be removed if the neurosurgeon deems a high risk to health. However, debulking the tumour may allow radiotherapy and chemotherapy to work more effectively due to less tumour cells (in a dense core). However, each dosage also poses a damaging risk to healthy brain tissue, though healthy tissue is able to repair itself in between sessions whereas the tumour cannot.
Last Updated: Jan 21, 2020