The mucosal immune system is comprised of anatomically remote and physiologically independent compartments that protect ocular, nasopharyngeal, respiratory, oral, gastrointestinal, and genitourinary mucosae. Collectively, the mucosal surface exceeds 300 m2.
Mucus. Image Credit: ilusmedical/Shutterstock.com
The mucosal immune system provides the first line of defense against toxic agents that enter the body through the mucosal membrane. It comprises the largest immune organ in the body and can be viewed as a single layer of epithelium covered by mucus, in which the anti-microbial proteins are reinforced by innate and adaptive immune strategies.
In addition to cells of the immune system, there is an extensive microbial community comprised of pathogenic, commensal, and symbiotic microorganisms’ parents among all the mucosal sites, the gastrointestinal microbiota comprises the largest anatomical concentration, with approximately 1012 bacteria per cm3.
The inductive and effector sites
The mucosal immune system is distinct and differentiated at each anatomical location; however, it can be divided into inductive and effective sites based on functional and anatomical properties.
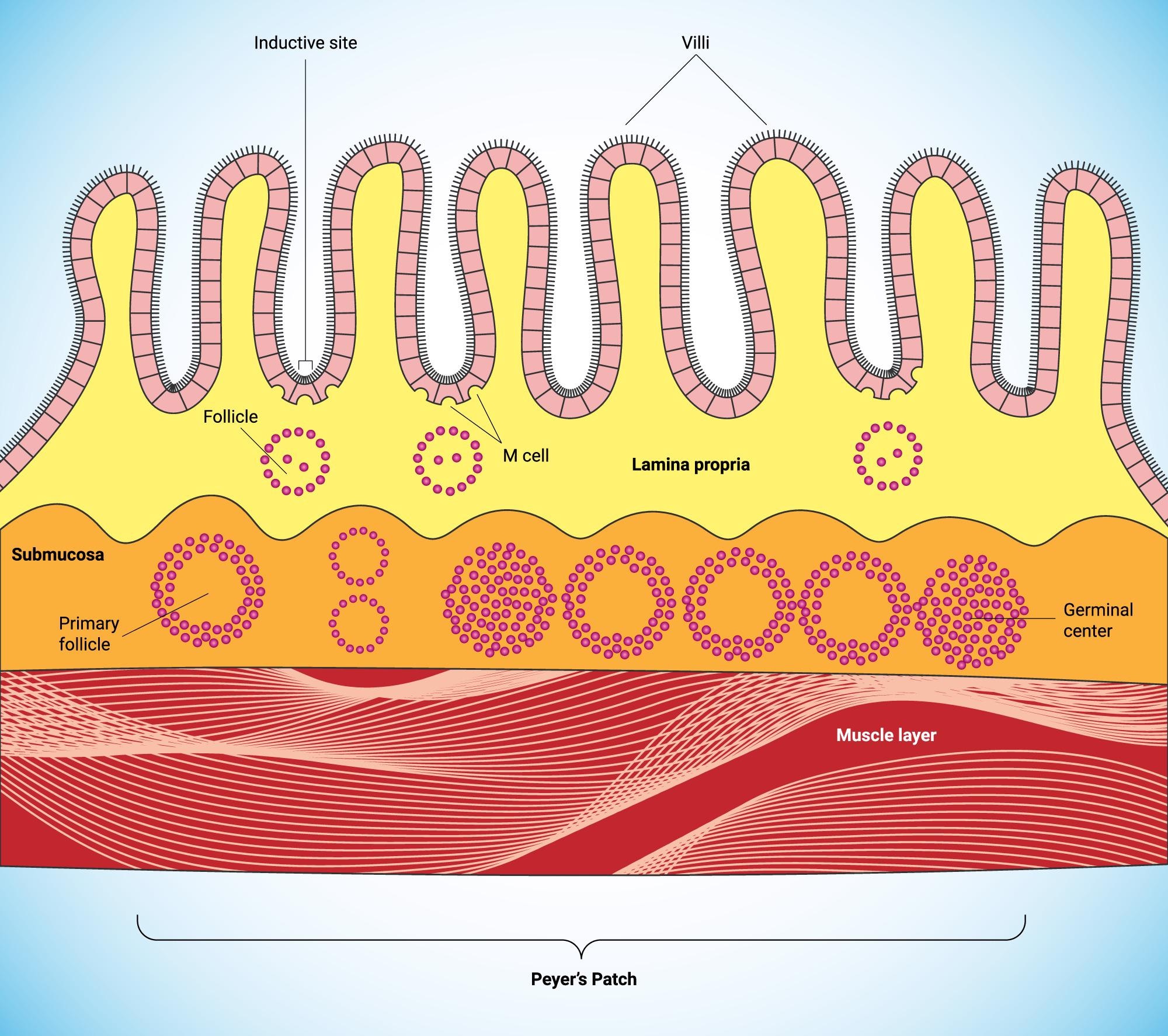
The mucosal inductive sites are comprised of mucosa-associated lymphoid tissue. Among them are the gut-associated lymphoid tissues (GALT), nasopharyngeal-associated lymphoid tissue (NALT), and lymphoid sites. The MALT is a source of memory B and T cells that migrate to effector sites to elicit immune responses.
The mucosal effector sites are comprised of the lamina propria regions of the upper respiratory, gastrointestinal, and reproductive tracts, alongside secretory glandular tissue (mammary and salivary). These effector sites contain antigen-specific mucosal effector cells; these include the TH cells, Treg cells, and IgA-producing plasma cells.
Secretory immunity
IgA can be selectively exported to external secretions as secretory IgA (SIgA). The induction of SIgA serves as the first line of defense in protecting the mucosal surfaces from enteric toxins and pathogenic microorganisms. SIgA promotes the clearance of antigens and pathogens via ‘immune exclusion’; IgA blocks their pathogenic access to epithelial receptors, resulting in mucus entrapment, and facilitating removal by mucociliary (apparatus of mucus and cilia which moves mucus along the epithelial surface for clearance) and peristaltic (relaxation of circular smooth muscles, then their contraction, mainly in the intestine) activities.
SIgA also demonstrates several other functions integral to mucosal homeostasis:
- Reinforcement of the epithelial barrier through multiple mechanisms
- SIgA-based immune complexes with pathogenic and/or commensal bacteria which are taken up by specialized M cells present in the epithelium where they are targeted to underlying dendritic cells which promote the downregulation of a local pro-inflammatory response
- Play a role in selection; SIgA excludes pathogenic bacteria from the epithelial surface by forming a biofilm of non-pathogenic bacteria as a result of anchoring within the mucus
Secretion of IgA at the effector sites occurs via an ∼80-kDa glycoprotein called polymeric Ig receptor (pIgR). pIgR is an epithelial membrane receptor that interacts with, and transcytoses, J chain-containing dimeric IgA (and pentameric IgM) produced by lamina propria plasma cells.
Around 80% of the antibody production of the body takes place locally in the gut lamina propria where the predominant form is the dimeric IgA ready for pIgR-mediated export. The total SIgA produced in adults is more than the total daily production of IgG in the body which demonstrates the clinical importance of IgA in the mucosae; most pathogens are encountered by the mucous membranes.
Strategies of the mucosal immune system
Role of oral tolerance
To maintain homeostasis, the mucosal immune system has evolved 2 adaptive anti-inflammatory mechanisms:
- Productive immunity: the first line of defense that is primarily mediated by secretory IgA and IgM antibodies in combination with various non-specific protective factors. This limits the colonization of the epithelial by pathogens as well as their penetration. Secretory immunity is stimulated by pathogens and the uptake of pathogens by the thin M cells (M) in the dome epithelium covering the MALT
- Suppression of pro-inflammatory responses: namely, the Th2-dependent responses (IgE antibodies), Th1-dependent delayed-type hypersensitivity (DTH), IgG antibodies, and Th17-dependent granulocytic reactions. ‘Oral tolerance’ regulates thus the process and the development of regulatory T (Treg) cells in mesenteric lymph nodes. Mucosal dendritic cells carry antigens to them and become conditioned for induction of Treg cells. This results in induced tolerance to food and other harmless antigens; other mechanisms of suppression may be involved. This oral tolerance explains the rarity of food hypersensitivity
The epithelial barrier
Mucosal tolerance is essential and provides a robust suppressive mechanism in response to the food that passes through the gut. Consistently, intact dietary antigens are taken up without causing harm. Essential to this function is the development during the neonatal period where priming for allergic disease occurs. During this developmental window, the epithelial barrier on immunoregulatory networks is vulnerable owing to their poor development.
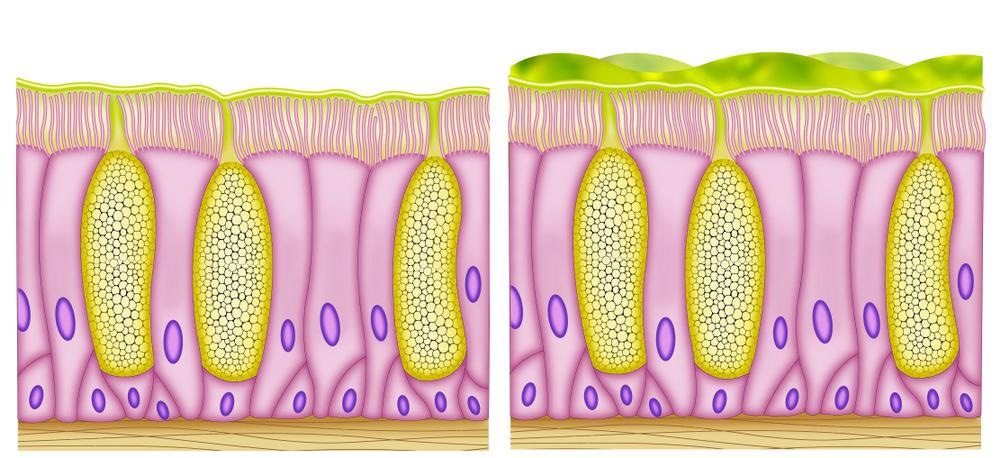
Structural components of the mucosal barrier. Image Credit: CLUSTERX/Shutterstock.com
Secretary immunity reinforces the epithelial barrier
Mucosal immunity differs from systemic immunity on a structural, cellular, molecular, and functional level. The intestinal mucosa is the home of more than 80% of the activated B cells which terminate differentiate to produce plasmablasts and plasma cells, making the intestine the largest antibody-producing organ.
Mucosal plasma cells typically produce dimeric IgA; alongside pentameric IgM, it is exported via the pIgR Present on the basolateral face of the secretory epithelial cells. In newborn babies and those with deficiencies in IgA, IgM antibodies play greater importance in the protective function of the mucosal immune system.
Overall, the mucosal immune system fulfills 3 functions: (1) protection of the mucous membrane against colonization and invasion; (2) prevent the uptake of undegraded antigens, including those from food, airborne matter, and those presented by commensal microorganisms and (3) to prevent the development of potentially harmful immune responses to exhaustion as antigens when they do make it past the epithelial barrier.
The mucosal immune system is unique in that it functions in a ‘dirty’ environment relative to the systemic immune apparatus. The immune system also selects the appropriate effective response and plays an essential role in regulating its intensity to prevent surrounding tissue damage and exhaustion of the systemic immune system.
References
- Mantis NJ, Rol N, Corthésy B. (2011) Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. doi:10.1038/mi.2011.41.
- Brandtzaeg P. (2009) Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. doi: 10.1111/j.1365-3083.2009.02319.x.
- Kabat AM, Pott J, Maloy KJ. (2016) The Mucosal Immune System and Its Regulation by Autophagy. Front Immunol. doi:10.3389/fimmu.2016.00240.
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The mucosal immune system. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27169/
Further Reading
Last Updated: Feb 21, 2022