What is neuroinflammation?
Causes of neuroinflammation
The impact of neuroinflammation
Potential treatments and prevention
References
What is neuroinflammation?
Neuroinflammation refers to an inflammatory reaction in the brain or spinal cord. It is driven by the production of molecules such as cytokines, chemokines, reactive oxygen species (ROS), and secondary messengers, which are released by endothelial cells, immune cells from the peripheral system, and resident glial cells in the central nervous system (CNS), such as microglia and astrocytes. 1
This process has widespread effects on the brain's immune, physiological, metabolic, and psychological functions. The severity and outcome of neuroinflammation depend on factors like the nature of the initial trigger, its duration, and how inflammation progresses. For example, it can lead to tissue damage, swelling, immune cell activation, and, in severe cases, cell death. 1
Although neuroinflammation has a protective role in defending the brain from harmful agents and assisting in tissue repair, it can become problematic when it is prolonged or excessive. Factors like genetic mutations, protein aggregation, infections, trauma, and certain drugs can all trigger sustained neuroinflammation, which in turn can hinder regeneration and contribute to neurodegenerative diseases. 2
The CNS is made up of neurons and glial cells, with the latter group—including astrocytes, oligodendrocytes, and microglia—exhibiting greater diversity and function than neurons. Once believed only to support neurons, glial cells are now known to play critical roles in regulating neuronal activity and innate immune responses. Microglia and astrocytes, in particular, can exhibit either neurotoxic or neuroprotective phenotypes, depending on the circumstances. 2
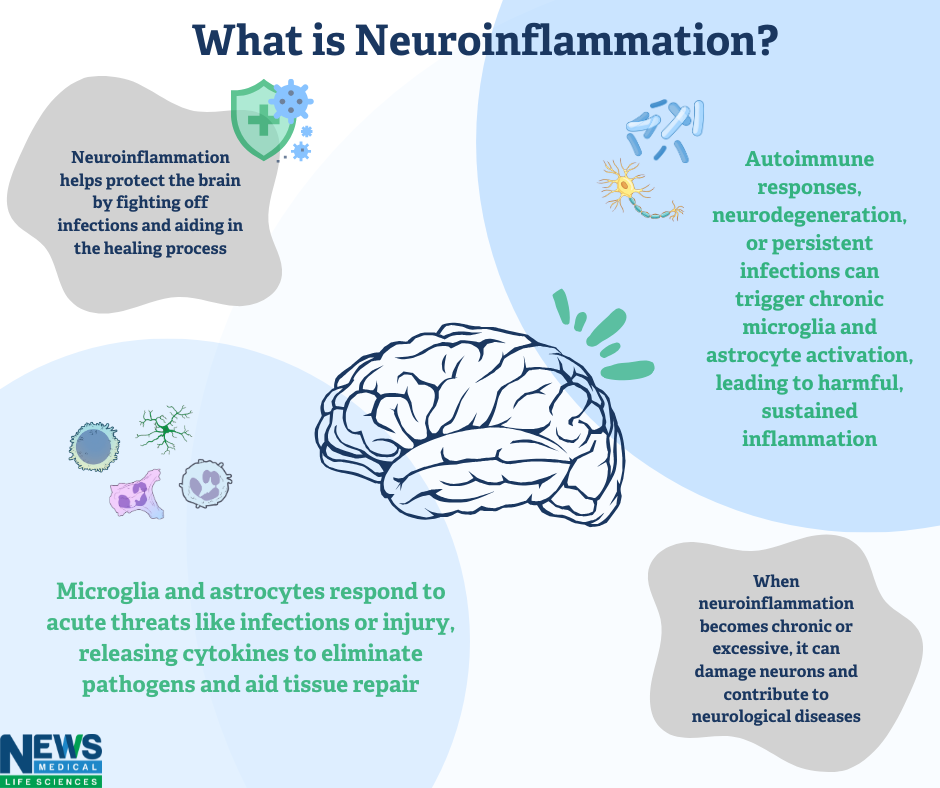
Image Credit: News-Medical.net
Causes of neuroinflammation
Neuroinflammation can arise from various triggers, including infections, systemic inflammation, autoimmune disorders, and lifestyle factors.
- Infections and peripheral inflammation: Viral infections in the CNS3, as well as chronic conditions like joint pain4 or gut inflammation5, are significant contributors to neuroinflammation.
- Autoimmune disorders: Autoimmune conditions, such as paraneoplastic limbic encephalitis (PLE), show how the immune system can mistakenly attack neural tissues. In cancers like small cell lung cancer, immune cross-reactions with onconeural antigens can damage neural tissue, triggering neuroinflammation. 6, 7
- Lifestyle factors: Poor diet can disrupt gut microbiota, alter blood-brain barrier (BBB) permeability, and promote neuroinflammation8, 9. Obesity, metabolic syndrome, and diabetes accelerate neuronal metabolism, producing ROS that leads to oxidative stress and inflammation. 10, 11
- Stress and sleep deprivation: Psychological stress increases cytokines such as TNF-α and IL-1, contributing to neuroinflammation and conditions like depression and anxiety. Similarly, sleep deprivation has been linked to increased neuroinflammation. 12, 13
- CNS injuries: Brain and spinal cord injuries lead to significant neuroinflammation, marked by glial activation (microglia and astrocytes), cytokine and chemokine production, immune cell infiltration, and increased BBB permeability. 14 Both penetrating and non-penetrating injuries trigger an acute inflammatory response that is beneficial for repair but harmful if prolonged. Secondary and chronic inflammation after these injuries often leads to long-term complications. 15, 16
Neuroinflammation can even occur without underlying pathology, highlighting the need to better understand its mechanisms for prevention and treatment.
The impact of neuroinflammation
Pathological neuroinflammation involves the activation of CNS glial cells (microglia and astrocytes), leading to the release of cytokines and chemokines, increased BBB permeability, and immune cell infiltration. This can result in tissue damage, ischemia, and cell death, often exacerbated by infections, injuries, or autoimmune conditions. 17,18
In diseases like multiple sclerosis (MS) and Alzheimer's disease (AD), neuroinflammation plays a central role in tissue destruction. In MS, chronic inflammation leads to demyelination and axonal loss, contributing to progressive disability. 19 In AD, glial activation and protein misfolding result in neuronal damage and cognitive decline. 20 These examples demonstrate how sustained neuroinflammation can lead to irreversible damage and long-term functional impairments. Research increasingly points to pro-inflammatory microglia as a key factor in neuronal damage, even though many cells contribute to neuroinflammation in Parkinson's disease (PD). 21,22
Although neuroinflammation initially serves a protective role, chronic and excessive activation in conditions like MS and AD leads to significant neurological damage and disability. The activation of microglia and astrocytes, along with the release of cytokines, chemokines, and neuroactive substances, enhances neuronal excitability and increases pain sensitivity. As a result, the interaction between the nervous and immune systems plays a key role in amplifying pain, driving neuroplastic changes, and contributing to chronic pain, as well as its emotional and cognitive impacts. 23
Potential treatments and prevention
To reduce neuroinflammation in PD, a customized, integrated approach that includes medication, non-pharmacological lifestyle modifications, and natural therapies targeting inflammation will likely be needed. Targeting microglia phenotypes to reduce harmful pro-inflammatory effects and boost neuroprotective anti-inflammatory activities may offer promising therapeutic options. 24
Image Credit: Billion Photos/Shutterstock.com
For Alzheimer's disease (AD), addressing neuroinflammation in the preclinical stage—before significant neuronal loss occurs—could be an effective treatment approach. While some Phase I/II clinical trials targeting TNF-α, TREM2, and CD33 have shown encouraging results, outcomes are still debated. Most clinical trials, including those examining anti-inflammatory substances, have not succeeded. Extensive longitudinal investigations with precise clinical and biomarker-based characterizations are necessary to identify efficient anti-inflammatory targets and treatments for AD. 25
Future clinical studies should focus on drug co-development pipelines and multimodal biomarkers. Promising developments in nanoparticle manufacturing technology could lead to new theranostic tools for controlled and targeted delivery of medication to neuroinflammatory regions. One promising approach involves creating biomimetic, cell-targeted, and stimuli-responsive multifunctional nanoparticles. 26
References
- DiSabato, D. J., Quan, N., & Godbout, J. P. (2016). Neuroinflammation: the devil is in the details. Journal of neurochemistry, 139 Suppl 2(Suppl 2), 136–153. https://doi.org/10.1111/jnc.13607
- Kwon, H.S., Koh, SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 9, 42 (2020). https://doi.org/10.1186/s40035-020-00221-2
- Klein R. S., Garber C., Funk K. E., Salimi H., Soung A., Kanmogne M., et al. (2019). Neuroinflammation During RNA Viral Infections. Annu. Rev. Immunol. 37 73–95. 10.1146/annurev-immunol-042718-041417
- Schrepf A., Kaplan C. M., Ichesco E., Larkin T., Harte S. E., Harris R. E., et al. (2018). A multi-modal MRI study of the central response to inflammation in rheumatoid arthritis. Nat. Commun. 9:2243. 10.1038/s41467-018-04648-0
- Do J., Woo J. (2018). From Gut to Brain: Alteration in Inflammation Markers in the Brain of Dextran Sodium Sulfate-induced Colitis Model Mice. Clin.Psychopharmacol. Neurosci. 16 422–433. 10.9758/cpn.2018.16.1.422
- Bien C. G., Vincent A., Barnett M. H., Becker A. J., Blumcke I., Graus F., et al. (2012). Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 135 1622–1638. 10.1093/brain/aws082
- Zhang H., Zhou C., Wu L., Ni F., Zhu J., Jin T. (2013). Are onconeural antibodies a clinical phenomenology in paraneoplastic limbic encephalitis? Mediators Inflamm. 2013:172986. 10.1155/2013/172986
- Dutheil S., Ota K. T., Wohleb E. S., Rasmussen K., Duman R. S. (2016). High-Fat Diet Induced Anxiety and Anhedonia: Impact on Brain Homeostasis and Inflammation. Neuropsychopharmacology 41 1874–1887. 10.1038/npp.2015.357
- Spielman L. J., Gibson D. L., Klegeris A. (2018). Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 120 149–163. 10.1016/j.neuint.2018.08.005
- de Heredia F. P., Gomez-Martinez S., Marcos A. (2012). Obesity, inflammation and the immune system. Proc. Nutr. Soc. 71 332–338. 10.1017/S0029665112000092
- Van Dyken P., Lacoste B. (2018). Impact of Metabolic Syndrome on Neuroinflammation and the Blood-Brain Barrier. Front. Neurosci. 12:930. 10.3389/fnins.2018.00930
- Curcio G., Ferrara M., De Gennaro L. (2006). Sleep loss, learning capacity and academic performance. Sleep Med. Rev. 10 323–337. 10.1016/j.smrv.2005.11.001
- Hurtado-Alvarado G., Domínguez-Salazar E., Pavon L., Velázquez-Moctezuma J., Gómez-González B. (2016). Blood-Brain Barrier Disruption Induced by Chronic Sleep Loss: Low-Grade Inflammation May Be the Link. J. Immunol. Res. 2016:4576012. 10.1155/2016/4576012
- Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053.
- Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18. doi: 10.3389/fneur.2013.00018
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4.
- Michael BD, Griffiths MJ, Granerod J, et al. The interleukin-1 balance is associated with clinical severity, blood-brain barrier permeability, neuroimaging changes and outcome in encephalitis. J Infect Dis. 2015 doi: 10.1093/infdis/jiv771
- Lovas G, Szilagyi N, Majtenyi K, Palkovits M, Komoly S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain. 2000;123(Pt 2):308–317. doi: 10.1093/brain/123.2.308.
- Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, Shepherd CE. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer's disease. Brain Pathol. 2009;19:392–398. doi: 10.1111/j.1750-3639.2008.00188.x.
- Saitgareeva, A. R., Bulygin, K. V., Gareev, I. F., Beylerli, O. A., & Akhmadeeva, L. R. (2020). The role of microglia in the development of neurodegeneration. Neurological Sciences, 41, 3609-3615.
- Nakagawa, Y., & Chiba, K. (2014). Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals (Basel, Switzerland), 7(12), 1028–1048. https://doi.org/10.3390/ph7121028
- Vergne-Salle, P., & Bertin, P. (2021). Chronic pain and neuroinflammation. Joint bone spine, 88(6), 105222. https://doi.org/10.1016/j.jbspin.2021.105222
- Kip, E., & Parr-Brownlie, L. C. (2022). Reducing neuroinflammation via therapeutic compounds and lifestyle to prevent or delay progression of Parkinson's disease. Ageing research reviews, 78, 101618. https://doi.org/10.1016/j.arr.2022.101618
- Liu, P., Wang, Y., Sun, Y., & Peng, G. (2022). Neuroinflammation as a Potential Therapeutic Target in Alzheimer's Disease. Clinical interventions in aging, 17, 665–674. https://doi.org/10.2147/CIA.S357558
- Cerqueira, S. R., Ayad, N. G., & Lee, J. K. (2020). Neuroinflammation Treatment via Targeted Delivery of Nanoparticles. Frontiers in cellular neuroscience, 14, 576037. https://doi.org/10.3389/fncel.2020.576037
Further Reading
Last Updated: Nov 22, 2024