Scientific studies performed on highly pathogenic viruses like the novel severe acute respiratory coronavirus 2 (SARS-CoV-2) virus must be carried out in laboratories that meet strict biosafety level (BSL) requirements.
.jpg)
Image Credit: Kateryna Kon/Shutterstock.com
Unfortunately, the requirements of BSL-3 and BSL-4 labs often prevent more than a few specific institutions from handling these agents, thereby hindering vaccine development efforts. The pseudovirus system is a useful alternative approach that can effectively screen vaccines on pathogenic viruses outside of a BSL-3 or BSL-4 level laboratory.
Defining a pseudovirus
The first documented use of the term “pseudovirus” was in 1967, wherein researchers described a particle that had been produced from cultured mouse cells that were infected by polyomavirus.
Upon further analysis of this particle, the researchers found fragments of mouse DNA to be encapsulated within the protein coat of the polyomavirus. Compared to a live virus, pseudoviruses, which can be either be naturally produced during an infection or artificially in a laboratory for research purposes, contain fragments of host-cell DNA without containing any of the nucleic acid components of the infectious virus to which they are related.
The modified genetic material of pseudoviruses prevents these particles from producing viral surface proteins on their own unless an additional plasmid or stable cell line that expresses such proteins are made available to the pseudovirus.
The different ‘pseudos’ in virology
It is important to identify the distinctions that exist between pseudoviruses and other virology terms that also utilized the word ‘pseudo’, such as orphan pseudovirus and a pseudotype virus.
Orphan pseudovirions, for example, contain fragments of the host cell’s genetic material that is encapsulated within a protein structure that closely resembles that of bacterial proteins.
To date, the protein structures present on the surface of orphan pseudovirion particles are not the gene product of any known infectious virus. Comparatively, the pseudotype virus has the genome of a known virus but is instead encapsulated with the protein coat of another type of virus.
Utility of pseudoviruses
Upon entry into susceptible cells, pseudoviruses are only capable of replicating once, which is comparable to wild-type (WT) viruses that often replicate multiple times. Additionally, pseudoviruses also lack the virulent components of their parent virus, which practically eliminates the possibility that these virus particles could cause an active infection to an exposed individual.
These unique properties of pseudoviruses allow for them to be safely handled in biosafety level (BSL) 2 laboratories, which typically work with agents that pose a moderate health hazard to humans. Although these viruses are much safer to handle than the virus from which they originated, the conformational structure of the surface proteins of pseudoviruses closely resembles that of the native virus. Such similarities in the surface protein structure allow pseudoviruses to remain effective in their ability to enter cells.
In addition to these advantages, the pseudovirus system is quantifiable and can be rapidly produced. The numerous benefits associated with performing scientific studies on pseudoviruses have assisted researchers during the detection of antibodies, the development, and evaluation of vaccines, as well as provide information on receptor recognition, virus inhibition pathways, and cellular tropism mechanisms.
Pseudovirus systems for SARS-CoV-2
Several recent attempts have been made to generate a reliable pseudovirus system for the SARS-CoV-2 virus that first appeared in 2019 and caused a pandemic.
One packaging system that has been used for the generation of SARS-CoV-2 pseudoviruses includes the highly efficient lentiviral vector system. Lentiviral vectors, which are typically the first packaging system used by researchers to create a novel envelope-pseudotyped virus, typically originating from human immunodeficiency virus (HIV-1), simian immunodeficiency virus (SIV), feline immunodeficiency virus (FIV) or vesicular stomatitis virus (VSV).
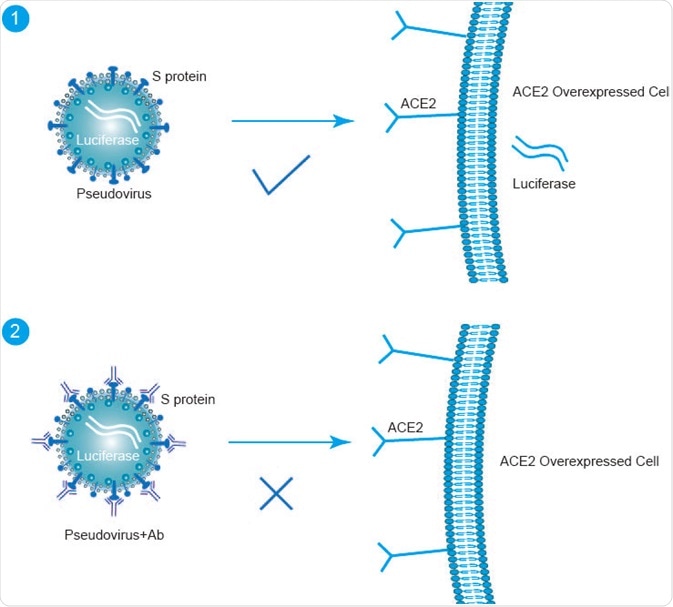
Image Credit: creative-diagnostics.com
VSV system
In one study, a group of Taiwanese researchers utilized the VSV lentivirus vector system to create a SARS-CoV-2 pseudovirus that expressed full-length S protein sequences.
In their work, the SARS-CoV-2 pseudovirus successfully entered the target cells while simultaneously exhibiting a much lower biosafety risk as compared to the native SARS-CoV-2 virus. This SARS-CoV-2 pseudovirus was also found to be potent for both antigenic property and immunogenicity analyses.
Using the SARS-CoV-2 pseudovirus, a pseudovirus-based neutralizing assay (PBNA) for the evaluation of potential therapeutic neutralizing antibodies was successfully generated.
In addition to being a much safer option as compared to a live virus assay, the PBNA developed in this study was found to be versatile, sensitive, accurate, reproducible, and robust, thereby making this assay extremely useful in the development and evaluation of antiviral candidate products.
HIV-1 system
In addition to the VSV system, several scientists have turned to the HIV-1 backbone for generating a SARS-CoV-2 pseudovirus. For example, in 2020, an American company introduced a novel SARS-CoV-2 pseudovirus luciferase assay (PVLA), which was created with an HIV lentiviral vector, that can also be applied for the evaluation of prospective drug candidates.
References
- Li, Q., Liu, Q., Huang, W., Li, X., & Wang, Y. (2018). Current status on the development of pseudoviruses for enveloped viruses. Reviews in Medical Virology 28(1). doi:10.1002/rmv.1963.
- “Do You Know the Difference in Laboratory Safety Levels 1, 2, 3 & 4s?” – Consolidated Sterilizer Systems
- Huang, S., Tai, C., Hsu, Y., Cheng, D., et al. (2020). Assessing the application of a pseudovirus system for emerging SARS-CoV-2 and re-emerging avian influenza virus H5 subtypes in vaccine development. Biomedical Journal. doi:10.1016/j.bj.2020.06.003.
- Nie, J., Li, Q., Wu, J., Zhao, C., et al. (2020). Establishment and validation of a pseudovirus naturalization assay for SARS-CoV-2. Emerging Microbes & Infections 9; 680-686. doi:10.1080/22221751.2020.1743767.
- Nie, J., Li, Q., Wu, J., Zhao, C., et al. (2020). Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nature Protocols 15; 3699-3715.
- “SARS-CoV-2 Pseudovirus Neutralization Assay” – Creative Diagnostics
Further Reading
Last Updated: Mar 5, 2021