What is the TME?
Components of TME
The TME and tumor-proliferation
TME and gut microbiota
Clinical role of TME
References
Further reading
For a prolonged period, cancer was thought to be a genetic disease where mutations spur malignant changes in the cell. In recent years, the growth in knowledge about the tumor microenvironment (TME) has altered this view. This article briefly introduces the TME and discusses its role in cancer progression.
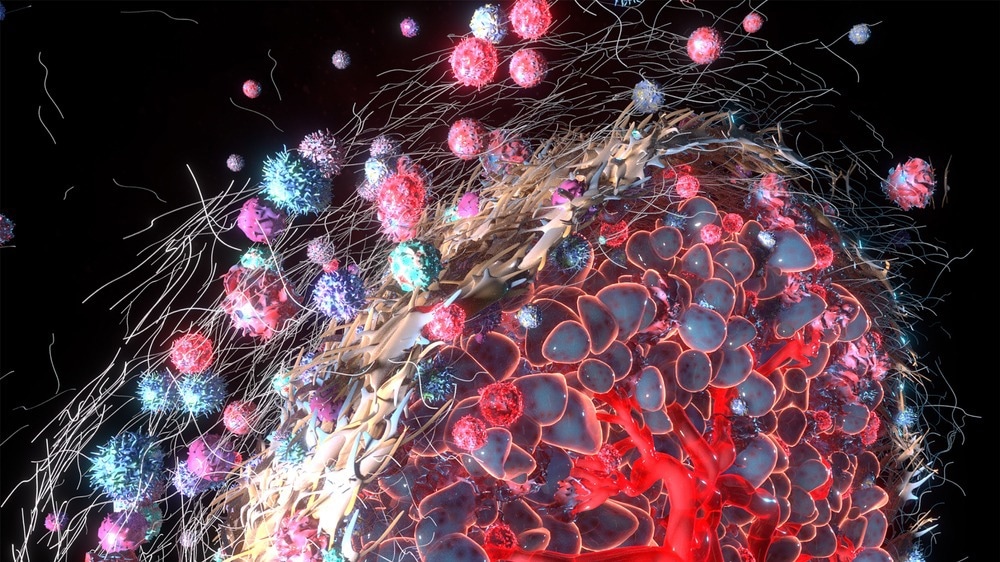
Image Credit: Alpha Tauri 3D Graphics/Shutterstock.com
What is the TME?
A tumor is a complex and heterogenous ecosystem, which not only contains malignant cells but several other interacting cells (e.g., stromal fibroblasts, endothelial cells, and a variety of immune cells) that controls its growth. TME refers to the cells, molecules, and blood vessels surrounding and feeding a tumor cell. It represents a non-malignant region around the tumor cell.
TME contains specific features, such as the varying degree of tumor infiltration by cytotoxic T cells, which can be used to predict a patient’s clinical outcome. Recent studies on renal cell cancer underscored the importance of determining the phenotype of the infiltrating T cells for predicting early relapse.
Components of TME
Although the composition of the TME is very specific to the tumor type, several characteristic features are also common to all TMEs. These comprise immune cells, blood cells, stromal cells, and the extracellular matrix. The TME is a continually evolving complex microenvironment constantly interacting with tumors; hence, one can and does affect the other.
Immune cells are an essential component of the TME, and they serve to either promote or suppress carcinogenic growth. Almost all immune cell types, such as polymorphonuclear cells, macrophages, dendritic cells (DCs), natural killer (NK) cells, and T and B lymphocytes, can infiltrate cancer tissues.
Immune cells are divided into the adaptive immune response and the innate response. The specialized cells (T cells, B cells, and NK cells) and antibodies of the adaptive immune system defend the human body by attacking and destroying foreign bodies.
Here, immunological memory is deployed so that these foreign invaders are remembered, and a stronger immune response is mounted upon reinfection to prevent the re-occurrence of the disease.
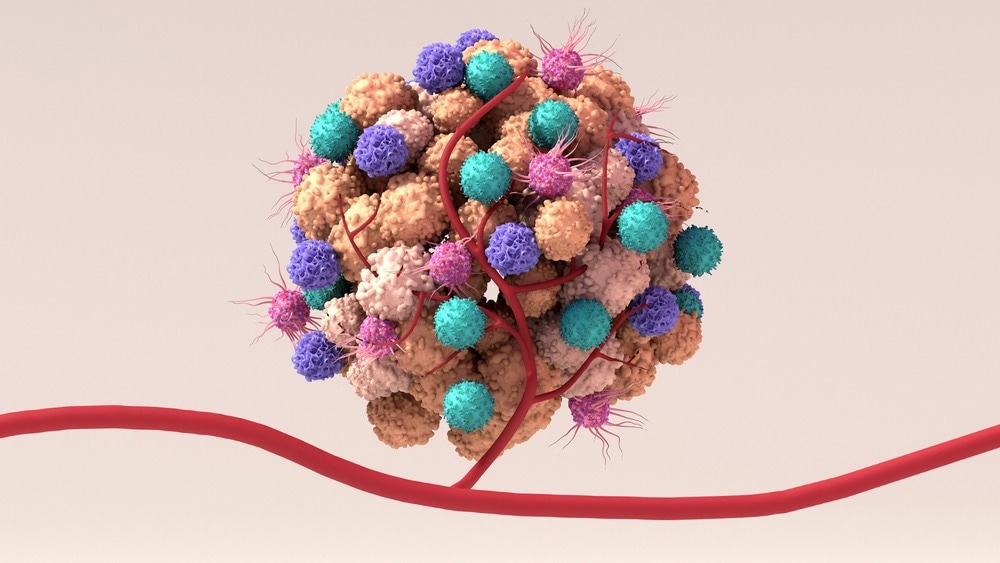
Image Credit: Design_Cells/Shutterstock.com
The innate immune response, meanwhile, is non-specific. It springs into action within hours of invasion by a foreign body or antigen. The cells involved in this kind of immunity are macrophages, neutrophils, and DCs.
The TME and tumor-proliferation
The proliferation, angiogenesis, cell recruitment, and immune suppression processes in the TME are the hallmarks of cancer. Within the complex and dynamic environment of the TME, cancer cells interact with stromal cells (differentiating cells that formulate the connective tissue of any organ of the body) via a plethora of biochemical and physical signals integral to tumor growth and spread.
Advances in tumor biology have demonstrated that analysis of the communication or ‘cross-talk’ between the tumor and the TME is crucial to our understanding of tumor growth and metastasis mechanisms. The tumor uses complex signaling networks to control the function of cellular and non-cellular components. Normal cells (i.e., non-tumor cells) are hijacked by the tumor at the core of the TME.
The TME is immunosuppressive. This means that the tumor is often able to proliferate by evading the body’s immune system and subverting drug therapy. Once the TME becomes established, it continues to present a robust barrier to the immune system.
The Tumor Microenvironment
The tumor itself mounts a defense by producing key inhibitory cells and molecules, death factors being one good example, and actively downregulating an anti-tumor immune response. Other defense mechanisms in the TME include disruption to normal tissue homeostasis.
Tumor cells require sufficient oxygen and nutrient levels to support their growth as they have high metabolic and mitotic rates. A vascular network is mandatory for tumor development. The production of proangiogenic factors, such as transforming growth factor-β, vascular endothelial growth factor, platelet-derived growth factor, and fibroblast growth factor, has been associated with a hypoxic tumor environment. These factors are responsible for aberrant vasculature.
The rapid growth of the tumor at the core of the TME can outstrip the supply of oxygen availability. This, in addition to impairments in blood supply to the tumor, creates a hypoxic environment. To beat this inhospitable situation, the TME orchestrates an increase in oxygen and nutrient levels (via angiogenesis –the promotion of new blood vessels) and removes toxic waste. This exacerbates the risk of metastasis and tumor survival rate, and encourages the suppression of anti-tumor immunity.
TME and gut microbiota
Recently, the role of microbiota in enhancing the tumor growth rate and spread has been documented. Mechanistically, the gut microbes are involved with the shaping of the systemic and in situ immune response.
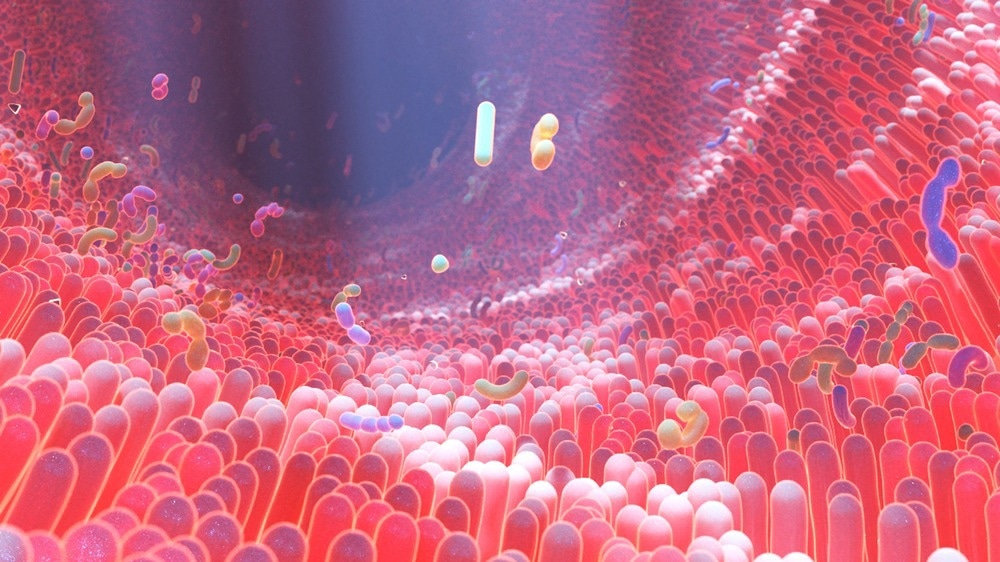
Image Credit: Alpha Tauri 3D Graphics/Shutterstock.com
Besides intestines, millions of microbes are also colonized in skin and mucosas, which interact with hosts constantly. These interactions have significantly shaped the functional diversity and the range of B and T cells. Interestingly, the role of these microbes is diverse. For instance, changes in gut microbiota could promote HIV disease progression. In some cases, microbes are also responsible for the systemic expansion of Treg, which is essential for preventing tissue inflammation.
According to a recent study, hepatocellular carcinoma is promoted by the intestinal microbiota through the activation of TLR4. Furthermore, an animal model indicated that radio-and chemotherapy caused gastrointestinal bacterial translocation into the systemic circulation, which enhanced immune response and increased post-therapy tumor rejection.
Clinical role of TME
Over the past decade, cancer treatment has undergone a revolution. Compared to traditional chemotherapy drugs of the past, which were non-specific and broad-ranging, current therapeutic strategies target specific cells within the TME.
Several studies have demonstrated that assessing the degree of tumor infiltration by cytotoxic T cells can predict the clinical outcome in melanoma, ovarian, and colorectal cancer patients. These studies have also indicated that endothelial cells and fibroblasts can modulate the clinical impact of immune cells in the TME.
The recent evidence strongly highlighted that cellular and acellular components of TME could reprogram tumor initiation, invasion, growth, metastasis, and response to therapies. Considering the significance of TME in cancer biology, scientists have focused on a TME-centric treatment model. In the future, more research is required to realize and enhance the efficacy of the therapeutic strategies targeting TME.
References
- Farc, O. and Farc, O. (2021) An overview of the tumor microenvironment, from cells to complex networks (Review). Experimental and Therapeutic Medicine, 21(96). https://doi.org/10.3892/etm.2020.9528
- Jin, M.Z. and Jin, W.L. (2020) The updated landscape of tumor microenvironment and drug repurposing. Signal Transduction and Targeted Therapy, 5(166). https://doi.org/10.1038/s41392-020-00280-x
- Anderson, N. (2020) The tumor microenvironment. Current Biology. (Online) https://www.cell.com/current-biology/pdf/S0960-9822(20)30933-7.pdf.
- Baghban, R. et al. (2020) Tumor microenvironment complexity and therapeutic implications at a glance. Cell Communication Signal. doi: 10.1186/s12964-020-0530-4.
- NIH–National Cancer Institute (n.d.) ‘Tumor microenvironment’ (online): https://www.cancer.gov/publications/dictionaries/cancer-terms/def/tumor-microenvironment.
- Giraldo, N.A., et al. (2019) The clinical role of the TME in solid cancer. British Journal of Cancer, 120, pp. 45–53. https://doi.org/10.1038/s41416-018-0327-z
- Whiteside, T. (2008) The tumor microenvironment and its role in promoting tumor growth. Oncogene. doi: 10.1038/onc.2008.271.
Further Reading
Last Updated: Dec 13, 2022