Adenosine triphosphate (ATP) is a high-energy molecule present in living cells. It is a nucleoside triphosphate that provides energy within cells for metabolism and is used in several cellular processes including the synthesis of key biomolecules.
ATP is typically present in cells at a concentration of 1-10mM – it is continuously recycled in organisms to ensure there is a constant energy supply for cellular processes.
ATP can be synthesized by redox reactions that use simple and complex lipids or carbohydrates as the source of energy. Complex energy sources need to be digested into simpler molecules before being used in ATP synthesis. Complex carbohydrates are usually hydrolyzed into glucose and fructose, while triglycerides are metabolized to form glycerol and fatty acids.
The biosynthesis of ATP by oxidative phosphorylation and photophosphorylation is the fundamental pathway for energy production in animals, plants, and microbes. Eukaryotic ATP production usually takes place in the mitochondria of the cell.
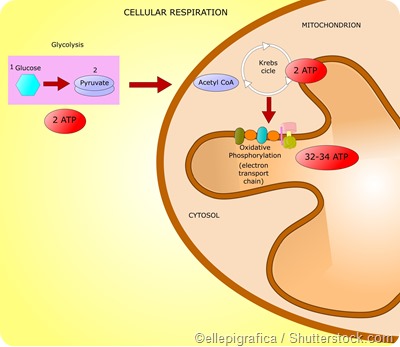
Important pathways by which eukaryotes generate energy are glycolysis, the citric acid cycle (or the Kreb’s cycle), and the electron transport chain (or the oxidative phosphorylation pathway).
Together these 3 stages are referred to as cellular respiration. In human beings, cellular respiration converts adenosine diphosphate (ADP) to ATP and thus releases energy from molecules that are rich in energy.
Glycolysis
Glycolysis involves the metabolization of glucose and glycerol to form pyruvate. These reactions take place in the cytoplasm in most organisms and release a net amount of 2 ATPs. Here glucose is converted to pyruvate via phosphorylation with the help of 2 key enzymes - phosphoglycerate kinase and pyruvate kinase.
The overall reaction can be represented as below:
Glucose + 2 NAD+ + 2 ADP + 2 Pi à 2 Pyruvate + 2 NADH + 2 H+ + 2 ATP + 2 H2O
Thus each molecule of glucose undergoes glycolysis to give 2 pyruvates. Two reduced nicotinamide adenine dinucleotide (NADH) molecules and 2 water (H2O) molecules are also produced as a result of glycolysis. The NADH molecules are oxidized in the electron transport chain to produce ATP and the pyruvate produced is used a substrate for the Kreb’s cycle.
Kreb’s cycle
The Kreb’s cycle is also known as the tricarboxylic acid (TCA) cycle and occurs in the mitochondria. It involves a series of reactions by which pyruvate is degraded into CO2, ATP, water, and electrons.
Pyruvate produced by glycolysis in the cytoplasm gets converted to acetyl-Coenzyme A (acetyl-CoA) in the mitochondria. The acetyl-CoA is converted into citrate which then undergoes a series of series of redox, hydration, dehydration, and decarboxylation reactions to form isocitrate, alpha-ketogluterate, succynl-CoA, fumerate, and malate.
These reactions are catalyzed by several key enzymes in the pathway such as citrate synthase, aconitase, isocitrate dehydrogenase, and malate dehydrogenase. Overall, the Kreb’s cycle yields 2 ATP molecules, 6 NADH molecules, and 2 reduced flavin adenine dinucleotide (FADH2) molecules.
Acetyl-CoA derived from the metabolism of carbohydrates, fats, and proteins in cells is all used in the Kreb’s cycle to generate energy. Therefore, this is an important metabolic pathway that unites carbohydrate, fat, and protein metabolism in living organisms.
NADH and FADH2 molecules generated as byproducts of the citric acid cycle are fed into the electron transport chain where they are oxidized to produce ATP with the help of the enzyme ATP synthase. This enzyme is present in the mitochondria and catalyzes the production of ATP by combining ADP and inorganic phosphate.
ATP synthase is often referred to as a complex molecular machine which has central rotor that moves at a speed of 150 rotations/s during ATP synthesis.
In total, each glucose molecule undergoing cellular respiration produces 38 ATP molecules – 2 ATPs from glycolysis, 2 ATPs from the Kreb’s cycle, and 34 ATPs from the electron transport chain.
References
- http://www.ncbi.nlm.nih.gov/pubmed/11997128
- http://www.ncbi.nlm.nih.gov/pubmed/12745923
- https://www.ncbi.nlm.nih.gov/pubmed/11997128
Last Updated: Feb 26, 2019