Marburg virus is a deadly pathogen that causes Marburg disease a severe viral hemorrhagic fever, named after the city in Germany, where the first outbreak occurred in 1967.
The virus has had several outbreaks across the world since then, with much research focus on its structure and transmission method due to its unusual nature.
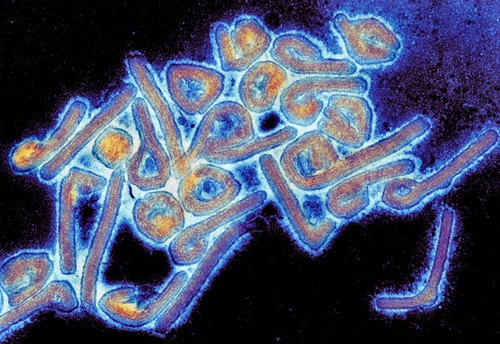
"Colorized Marburg virus particles viewed with a transmission electron microscope" by Microbe World is licensed under CC BY-NC-SA 2.0![]()
![]()
![]()
![]()
Marburg virus background and taxonomy
Marburg virus is part of the Filoviridae family, along with another zoonotic disease: ebola. Although it is a single species, different lineages of the Marburg virus differ from one another by up to 21% at the nucleotide level.
Compared to other viral species, the Marburg virus is quite slow to mutate and does not have a comparatively large variation between strains. The reason behind this is not well known, but it is of interest to researchers focusing on more rapidly evolving pathogens such as HIV.
Structure
Marburg virus has an unusual shape. They are pleomorphic in shape, which means they can be a number of different shapes are rod-like or ring-like, crook- or six-shaped, or with branched structures.
Research has indicated that around 30% of viral particles are filamentous, 37% are six-shaped, and 33% are round. This has been at odds with other research, which found that all Marburg virus particles were 80 nm in diameter (compared to the other study’s mean of 91 nm) but that they varied greatly in length.
The membrane of Marburg virus particles is derived from the host and coated with spikes made of the viral glycoprotein. These assist in attachment, receptor binding, and fusion. The glycoproteins are among the largest proteins in Marburg viral particles.
At the core of the viral particle is the ribonucleoprotein complex, also called the nucleocapsid, which is made up of the viral RNA genome and its associated nucleocapsid proteins. The nucleocapsids are tubular structures that remain tightly associated with the RNA genome and give it its helical shape.
Transmission
Marburg virus is a zoonotic disease, meaning it persists in an animal population and is then spread to humans. People infected with the Marburg virus from exposure to an infected animal will then pass it on to other humans.
The zoonotic nature of the Marburg virus means that there are certain hypothetical limits to where outbreaks can occur outside the laboratory. Its ecological area, where it has suitable hosts, are in certain areas of eastern, south-central Africa where the climate is dry and open.
For example, the outbreak in Angola was found to have been ecologically predicted, but it was the first occurrence that couldn’t be traced to eastern Africa. The original German outbreak came from imported monkeys.
Human to human transmission occurs via direct contact of bodily fluids, such as saliva, sweat, stool, and breast milk.
Image Credit: Mauro Rodrigues/Shutterstock.com
Research shows that the virus has been found in tears and semen, in addition to the liver even weeks or months after symptoms appear. However, research suggests that transmission of Marburg virus from an infected human is generally unlikely, except when in close contact without protective equipment such as during treatment without barrier nursing or certain burial practices.
Replication cycle
Studying the replication cycle of the Marburg virus is done stepwise, where versions of the virus that lack certain elements to stop them from becoming dangerous are used. While this offers insight into the entry, replication, and budding, they lack the morphological features and protein composition of real Marburg viral particles.
In general, Marburg virus entry into infected cells consists of attachment, endocytosis, and fusion. Both attachment and fusion are mediated by Marburg virus glycoprotein, where it binds to different types of C-type lectins or TAM receptor protein kinases. The method by which endocytosis occurs is not well understood but might occur similarly to the ebola virus, to which it is related.
Transcription and replication of the viral RNA genome occur after the nucleocapsid is released into the infected cell. Seven monocistronic mRNAs are transcribed and encapsidated by the nucleocapsid proteins.
The new nucleocapsids are subsequently recruited to sites where virus budding occurs. Glycoproteins are recruited to the budding sites, where VP40 induces the formation and release of filamentous virus-like particles.
Last Updated: Apr 1, 2020