Cancer cells have special metabolic features that contribute to survival, proliferation, and metastasis. A focal point for these metabolic changes is nicotinamide adenine dinucleotide (NAD+), where NAD+ levels in cells need to be balanced for processes to function effectively.
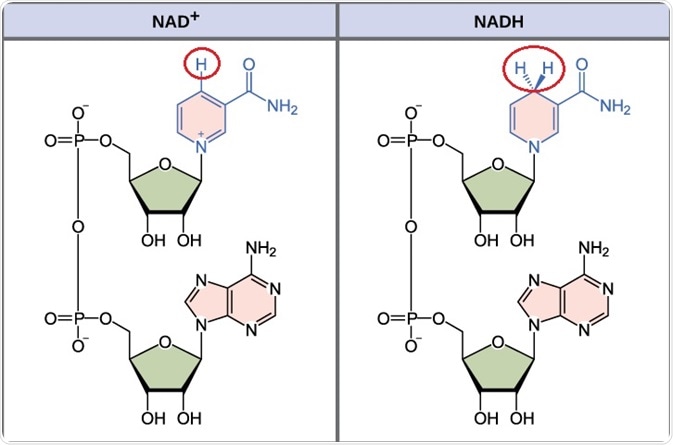
Image Credit: bioserendipity.com/anti-aging-supplements-ii/nad_nadh/
NAD+ metabolism
The most well-established function of NAD+ is in oxidation and reduction reactions. In addition to this, NAD+ is a substrate in biochemical reactions such as mono- and poly-ADP-ribosylation, protein deacetylation, and ADP-ribose cyclization.
These functions are particularly relevant for tumor cells because they have adaptations to allow for more active glycolytic, pentose, and fatty acid synthesis pathways. All of this means that NAD+ metabolism is involved in energy metabolism, repair of DNA, gene expression, and stress responses in cells. Increased levels of NAD+ boost glycolysis and allow for the quick proliferation of cancer cells.
Important enzymes in NAD+ biosynthesis
During these reactions, NAD+ is destroyed. For tumor cells, the high turnover rate of NAD+ is because of the high activity of ADP-ribosylation by PARPs (poly(ADP-ribose) polymerases). Critically low levels of NAD+ in cells causes metabolic collapse and death of the cell. Because of this, the tumor cells need to replenish lost NAD+ molecules via ingestion or biosynthesis.
Biosynthesis of NAD+ involves two important enzymes; Nampt and Nmnat. Nampt converts nicotinamide (NAM) and 5-phosphoribose-1-pyrophosphate (PRPP) to nicotinamide mononucleotide (NMN). From this, Nmnat creates NAD by moving a moiety of an adenylyl from ATP to NMN. Nampt is often overexpressed in several types of cancer, such as colorectal, ovarian, breast, and gastric tumors.
It has been found that levels of Nampt are upregulated in some cancers, implicating that it has a role in maintaining NAD+ levels in the cell. The inhibition of Nampt is associated with lethal reductions in NAD+ levels. However, Nampt is also needed in healthy cells, meaning any use in therapeutic settings needs to be thoroughly evaluated for deleterious side effects before use can be widespread.
NAD+ as a therapeutic target
The critical role NAD+ has in metabolism and cellular health has opened up the possibility of targeting it to kill off cancerous cells. This has been theorized to involve synthesis or degradation pathways, or both, which can deleteriously interrupt the cell’s normal functioning. This could involve, for example, using SIRT1 activation to increase NAD+ degradation or using Nampt inhibitors to prevent biosynthesis of NAD+.
There has been some success in identifying differences in NAD+ pathways of tumor cells and healthy cells, thereby allowing targeting only dangerous cells. FK866 is an inhibitor of Nampt in humans, and the subsequent reduction in NAD+ following its use can lead to apoptosis in tumor cells whereas normal healthy cells remain largely unharmed.
Despite this, as many as four-phase, I clinical trials of FK866 did not show any tumor remission in patients with advanced solid tumors. Other Nampt inhibitors, such as GMX1777 have shown similar effects to FK866.
Another potential target for NAD+ related therapy includes nicotinic acid/nicotinamide mononucleotide adenylyltransferase (Nmnat). This enzyme is involved in the production of nicotinic acid adenine dinucleotide (NAAD+), which then becomes NAD+.
Nmnat is central in catalyzing the conversion of tiazofurin, an anticancer drug, into its active form. In addition to this, many cancer cells have low Nmnat levels, except for Burkitt’s lymphoma and chronic myelogenous leukemia. Because of this, Nmnat has also been seen as a viable target for cancer therapies.
In addition to targeting the enzymes involved in NAD+ synthesis, some research has attempted to target the enzymes that consume NAD+. PARP is important for DNA repair, modifications in chromatin, cell transformations, and cell death. The BRCA mutation in certain breast cancers hinders the homologous recombination of DNA double-stranded breaks. This specific pathway has presented PARP as a potential therapeutic target.
PARP inhibitors can, in such cancers, increase the amount of single-strand breaks in DNA and thus lead to cell death. While direct PARP inhibitors have been investigated, a similar effect can be achieved by limiting the amount of available NAD+, since PARP uses NAD+ for its activity. Studies that have used a combination of PARP inhibitors and FK866 have found that this induced lethality in breast cancer cells.
.jpg)
Image Credit: crystal light/Shutterstock.com
Sources
- Mayo Clinic. 2020. Mayo Clinic Pancreatic Cancer SPORE - Targeting NAD Catabolism In Pancreatic Cancer Cells. [online] Available at: <https://www.mayoclinic.org/> [Accessed 6 August 2020].
- Khan, J., Forouhar, F., Tao, X. and Tong, L., 2007. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opinion on Therapeutic Targets, 11(5), pp. 695-705.
- Yaku, K., Okabe, K., Hikosaka, K., and Nakagawa, T., 2018. NAD Metabolism in Cancer Therapeutics. Frontiers in Oncology, 8, pp. 622.
- Kennedy, B., Sharif, T., Martell, E., Dai, C., Kim, Y., Lee, P., and Gujar, S., 2016. NAD+ salvage pathway in cancer metabolism and therapy. Pharmacological Research, 114, pp. 274-283.
Further Reading
Last Updated: Mar 13, 2023