The isoelectric point (pI) is the point at which the net charge on a molecule is zero. pI is most commonly examined for proteins. Each of the amino acids in a protein carries a distinct charge, and the overall charge of a protein is the summation of the individual charges on each amino acid. This charge is also dependent on the pH of the surrounding solution.
 Bacsica | Shutterstock
Bacsica | Shutterstock
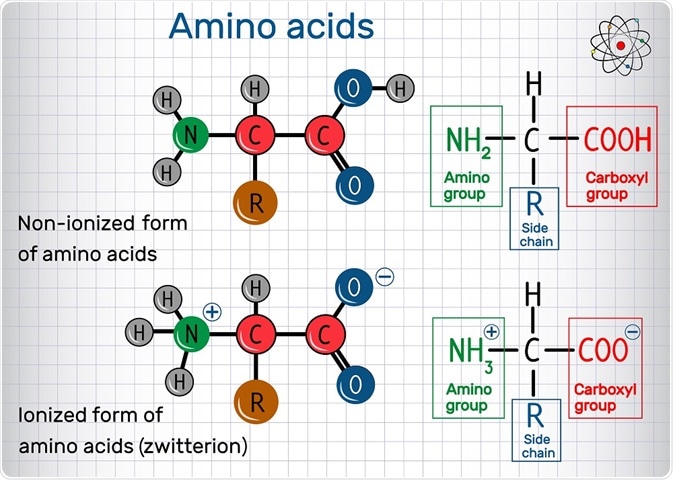
Zwitterions
The isoelectric point corresponds to what is known as the zwitterionic form of a protein. Zwitterion possesses discrete functional groups, each of which carry a positive or negative charge. A zwitterionic amino acid consists of a negative carboxylate ion and a positive ammonium ion alongside a charge on their side chain, which varies between amino acids.
What is the relationship between the pH and pKa of an amino acid?
The pKa for an amino acid is an acid dissociation constant that refers to the equilibrium between the protonated and deprotonated forms of the backbone amino group, backbone carboxyl group, and any potential acid/base component of the variable group. The net charge of the protein is determined by summing the charge of individual amino acids across the protein.
pKa relates to the equilibrium constant and defines the transition between two structural forms of an amino acid–protonated and deprotonated. Amino acids possess distinct pKas for the carbonyl group, amino group, and any functional groups on the side chain that may be protonated or deprotonated.
There is always one more structure than the number of pKa values for amino acids. For example, if there were two pKa values, three structures can be discerned. This is illustrated below using glycine, which has two pKa values – pKa 1 represents the (de)protonation of the carboxyl group and pKa2 represents the (de)protonation of the amine group:
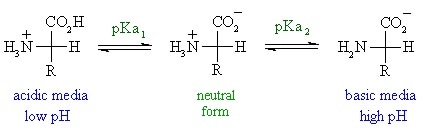
When the pH< pKa protonation of the amino and carboxyl groups occurs, resulting in a net +1 positive charge for glycine. When the pH is increased to a value between the two pKas(pKa2>pH> pKa1), the carboxyl group is deprotonated while the amino group remains protonated. This represents the zwitterionic form of the amino acid as it simultaneously possesses a positive and negative charge.
When the pH> pKa2, the amino group is deprotonated and loses its charge. The amino acid now carries a net negative charge of -1. The zwitterionic form of the amino acid can exist at any pH value between pKa1 and pKa2.
How does the zwitterionic form of an amino acid relate to the pI?
When the pH is exactly at the pKa value, a buffer arises in which the deprotonated and protonated amino acids exist in equilibrium. For example, when the pH = 2.34 (pKa of glycine), the solution comprises of 50% neutral molecules in which the carboxyl is deprotonated, and 50% positive molecules where the carboxyl is protonated.
This pH produces the carboxyl buffer zone. If the pH s increased to that of the pKa of the amino group (9.60), another buffer is produced where there is an equilibration between the protonated neutral zwitterion and the deprotonated negative amino acid.
The isoelectric point can, therefore, be approximated by averaging the two pKa values. More complex amino acids have more than two pKa values due to the presence of additional pKa values for their side chains.
The pI of amino acids with acidic side chains
In cases where the side chain is acidic, the pI is at a lower pH because the acidic side chain will result in an additional -1 charge. Subsequently, the neutral form arises under conditions of acidity, when the additional -1 charge has been neutralized. For example, aspartic acid has a pKa3 corresponding to its CH2CO2H side chain.
The pI of amino acids with acidic side chains
In case the side chain is basic, the pI is at a higher pH because the acidic side chain will result in an additional +1 charge. Subsequently, the neutral form arises under conditions of basicity, when the additional -1 charge has been neutralized.
Histidine persists in its neutral form between the pH of 6.00 (equivalent to pKa3 for the side chain pyrrole NH group, which corresponds to the equilibrium between the positive and neutral form) and pH 9.17 (equivalent to pKa 2 of the amino group which corresponds to the equilibrium between the negative and neutral forms).
Isoelectric focusing
One common application of pI is in a separation technique called isoelectric focusing which is based on the isoelectric point of a protein. A gradient of pH and electrical potential are applied across the gel.
One end of the gel is relatively positive, while the other is negative. When proteins are applied to the gel, they migrate through the gel and accumulate at the point along the pH gradient that corresponds to pI of the protein. As the net charge on the protein at this pH is zero, the protein can no longer migrate in the electric field.
Further Reading
Last Updated: Feb 14, 2019